The Time of Flight Mass Spectrometer
The
Time of Flight Mass Spectrometer (TOF)
was invented many years ago but, due to the factors controlling
resolution not being clearly recognized as a result of certain design
defects that occurred in the first models and also due to the
electronic components available at that time being significantly
inferior to those available today, it exhibited limited performance.
Consequently, the TOF was rapidly eclipsed by other developing mass
spectrometric techniques. However, with improved design, modern
fabrication methods and the introduction of Fourier transform
techniques, the performance has been vastly improved. As a result,
there has been a resurgence of interest in this particular form of
mass spectrometry. A diagram of the time of flight mass spectrometer
is shown in figure 80.
In
a Time of Flight Mass Spectrometer the
following relationship holds,
T = L((m/2zeV)0.5)
|
where (t)
|
is the time taken for the ion to travel
a distance (L)
|
|
(V)
|
is the accelerating voltage applied to
the ion,
|
|
and (L)
|
is the distance traveled by the ion to
the ion sensor.
|
It
follows that for a given system, the
mass of the ion is directly proportional to the square of the transit
time to the sensor. The sample is volatilized into the space between
the first and second electrodes and then a burst of electrons (over
about a microsecond period) is allowed to produce ions. The
extraction voltage, (E) is then applied for another short period of
time which, as those further from the second electrode will
experience a greater force than those closer to the second electrode,
this will focus the ions. The ions farther from the electrode will
experience a greater accelerating potential than those closer to the
electrode and, thus, will catch them up
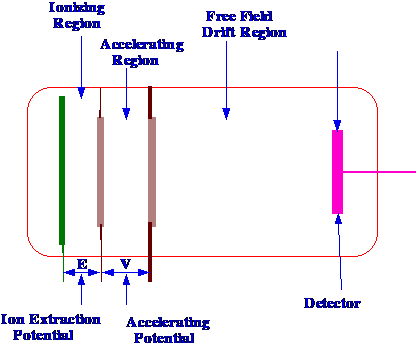
After
focusing, the accelerating potential
(V) is applied for a much shorter period than that used for ion
production (ca 100 nsec) so that all the ions in the
source
are accelerated almost simultaneously. The ions then pass through the
third electrode into the drift zone and are then collected by the
sensor electrode. One particular advantage of the time of flight mass
spectrometer lies in the fact that it is directly and simply
compatible with direct desorption from a surface. Consequently, it
can be employed with laser desorption and plasma desorption
techniques which have already been discussed.
The
first commercial Time-of-flight mass spectrometer was manufactured by
the Bendix Corporation, which was based on the original design of
Wiley McLaren. However, the model suffered from poor resolution
largely due to the relatively slow electronics that were available at
that time. In the 1970’s Kore Technology Ltd. revived the
instrument employing new designs and faster electronics. Over the
years as the speed of electronic systems became greater so did the
performance of the time-of-flight mass spectrometer and today the
instrument has a
wide field of
application in both research and analytical laboratories. The Proton
Transfer Reaction Time-of-Flight Mass spectrometer is shown in figure
80. Chemists interested in the detection and identification of trace
contaminants in the atmosphere find the TOF fast and efficient. The
Proton Transfer Reaction Time-of-Flight Mass spectrometer shown in
figure 80 was designed for the universities of Leicester and York and
employed proton transfer ionization source using H3O+
to transfer a proton to the analyst stream. This ionization procedure
ionized the molecules efficiently at the same time minimizing
molecular fragmentation. The major constituents of the atmosphere do
not react with H3O+
and so only the minor components are detected and measured. The TOF
system can detect atmospheric contaminants at levels down to one part
per trillion.
Courtesy
of Kore Technology Ltd.
The
instrument shown in figure 81
was developed by Kore for the Physics Department of Leicester
University to characterize the masses and distribution of metal
cluster produced by their proprietary metal cluster source.

Courtesy
of Kore Technology Ltd.
The beam of metal cluster particles pass
through the source region of the TOF-MS where they are ionized and
then orthogonally pulsed out into the mass spectrometer. Heavy masses
such as metal clusters have low velocities and consequently low
detection efficiencies. The effect is counteracted by floating the
detector at a high voltage to post-accelerate the ions, which
increases their velocity of impact and, consequently, the detection
efficiency.
As
a result of the basic layout of the TOF mass spectrometer and its
high sensitivity it can be designed in a compact shape that can be
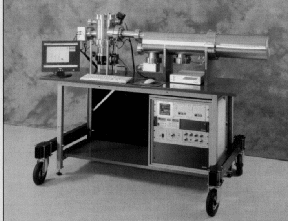
manufactured in a portable form. A photograph of Kore’s MS-200
portable mass spectrometer is shown in figure 82. As extremely small
quantities of sample are required, low powered, zero maintenance
vacuum pumps are used The MS-200 employs electron impact ionization
giving spectra that correspond well to established reference data.
The vacuum system is kept permanently sealed and contained in the
portable case. The vacuum is maintained by an ion pump and a
non-evaporable getter pump which can cope with all gases. Sample
gases are drawn though a heated inlet by a pump and enter the source
through a concentrating membrane inlet.

Courtesy
of Kore Technology Ltd.
Detection
limits are in the ppb range for
most compounds. Analysis time are about one minute at the highest
sensitivities. The general specifications of the mass spectrmeter
are,
Sensitivity
<
5 ppb in 10 seconds (Benzene)
Mass
range 1 –
1000 amu
Dynamic
Range 6 decades
Linearity
better than 5%.
An
excellent discussion on general organic
mass spectrometry is given in Practical Organic Mass
Spectrometry
edited by Chapman [13].

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.






