ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Examples of Special Applications
The Perkin Elmer Fluorescence
spectrometer was used in tandem with a Waters based liquid
chromatograph assembly, by Adams et
al. [4], to determine
Flurbiprofen and its major metabolite (4'-hydroxyflurbiprofen).
The sample matrixes were
physiological fluids such as blood serum or urine. The samples of
blood serum (100 μl)
were deprotenized with acetonitrile (1 ml) and buffered to a pH of
2.6 with 2 ml 0.05M potassium phosphate . The structural analogue,
2-(2-methoxy-4-biphenyl)propionic acid was used as an internal
standard. 100 μl
aliquots of the supernatant liquid were separated on a Waters
μBondepak
C18 column, using a mixture of 55% of 0.05M potassium phosphate (pH
2.6), and 45% tetrahydrofuran as the mobile phase.
The optimum excitation wavelength
was 260 nm and the emission wavelength that was monitored was 320 nm.
An example of the separation they obtained is shown in figure 12. It
is seen that an excellent separation is obtained and, as result of
the selectivity of the spectrometer operated at a the optimum
excitation wavelength, and monitoring at the optimum emission
wavelength, the peaks of interest are completely free from undetected
contaminant materials.
The recoveries of the drug and
metabolite ranged from 97.4% to 105.5%. The lower limit of detection
for the drug
(Flurbiprofen)
was approximately 1 x 10-6
g/ml, with a linear dynamic range extending to 50 x 10-6
g/ml. The linear range is small, but no less than would be expected
for Fluorescence measurements.
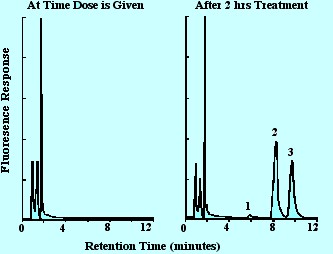
Excitation Wavelength
260 nm, emission wavelength 320 nm, 1. 4'-hydroxyflurbiprofen (metabolite), 2. 2-(2-methoxy-4-biphenyl)propionic acid
standard, 3. Flurbiprofin
Figure 12. Chromatogram of Flurbiprofin and its Metabolite from
Blood Serum Employing Selective Fluorescence Detection.
The same type of apparatus was used by Soroka et al. [5], to
determine a number of different metals as their fluorescent
8-hydroxyquinoline-5-sulfonic acid complexes. Nearly 80 different
metal species were examined and the optimum excitation wavelengths
and emission wavelengths for each was reported. The
8-hydroxyquinoline-5-sulfonic acid chelating reagent must be
carefully purified before use, to eliminate any trace of any
fluorescing materials, that would contribute background noise to the
measurements. An example of the separation of zinc , cadmium ,
magnesium and calcium complexes, by a chromatographic procedure, and
monitored by Fluorescence detection is shown in figure 13.
It is seen that for the metals separated, the optimum excitation and
emission wavelengths are very similar for all the metals
respectively, and seemed to be more a function of the characteristics
of the chelate, than those of the metals. The sensitivity of the
system indicated wide variation between metals, the minimum appearing
to be about 5 x 10-12
mol.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



