The Atmospheric Ionization Interface(API)
In atmospheric ionization, the
ions are
formed at atmospheric pressure by nebulization in the source region.
Ionization at atmospheric pressure has certain advantages. It avoids
the problems that arise when a liquid flows directly into a vacuum,
and, if used with some type of separation system, it allows it to
operate under ambient conditions. This is advantageous when low flow
rates are employed, such as with microbore columns and capillary
electrophoresis. A diagram of the atmospheric ionization interface is
shown in figure 45.

The atmospheric pressure
ionization (API)
process is similar in some respects to the electrospray ionization
source, and can cope with a range of column flow rates, up to a about
2 ml/min. Consequently, the total mobile phase can be utilized
without splitting the flow. However,
although all the solute may enter the interface, not all the solute
molecules are ionized, and not all the ions that are formed enter the
mass spectrometer. There are three forms of the
API
source: one uses a heated nebulizer with a corona discharge, another
employs an atmospheric electrospray and finally one
uses an
ion spray. The interface that employs a heated nebulizer and a corona
discharge ionization process is that depicted in figure 45. The
solvent is nebulized by a gas flow, which is then swept by a second
stream of gas (the sheath gas or make-up gas) through a quartz tube
heater and the solvent is vapourized. The sample then drifts through
a chamber containing a corona discharge (set up by a potential of
about 2000 volts, applied across a simple electrode arrangement). The
charged solvent vapor molecules are used as the ionizing agents (by a
Chemical Ionization process). The reactant ions, formed in the corona
discharge, collide with the sample molecules and give sample
molecule plus a proton (hydrogen
positive ions),
i.e. [M+H]+. The ions are
then drawn by means of
an electric field to a plate with an orifice, over which passes
another flow of gas called the curtain or barrier gas.
The
barrier gas helps to prevent uncharged molecules from entering the
ion source but the charged ions (due to the effect of the electric
field) pass through the orifice into the next chamber. The ions pass
through an aperture in the next plate (the skimmer plate) and the
space between the sampling plate, and the skimmer plate, is connected
to the first vacuum pump. Having passed through the skimmer plate,
the ions then pass through an aperture in a third plate into the mass
spectrometer analyzer. The space between the skimmer plate and the
final plate is connected to a second vacuum pump. By means of this
differential pumping, the necessary low pressure can be maintained in
the mass spectrometer. As the charged molecules entering the mass
spectrometer are virtually parent ions already, the fragmentation
pattern is similar to that obtained in MS/MS. In this way, the system
differs fundamentally from the electrospray
interface.
Ionization is soft, sensitive and gives good characteristic spectra
appropriate for sample identification and structural elucidation.
A recent modification of the API
technique
involved a low dead volume interface and was described by Thomson et
al. [29]. These workers employed packed microbore columns in
conjunction with a low-volume, wall-coated capillary column
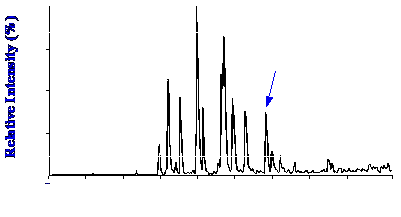
connections. The total ion current chromatogram of a tryptic digest
sample, comprising 1 picomole of human growth hormone, is shown in
figure 46. Flow rates of about 80 to 100 μl
per minute were used, with about 3 μl
passing to the capillary column and entering the interface.
Courtesy
of the Perkin Elmer SCIEX Corporation
It is seen that a good
separation is
obtained and apparently with little resolution lost in the capillary
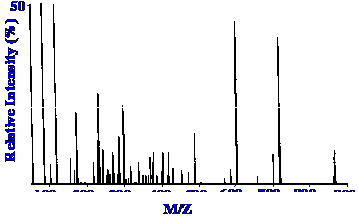
interface. The mass Spectrum of the peak marked T2 in the
chromatogram is shown in figure 47.
Courtesy
of the Perkin
Elmer SCIEX Corporation
Good spectra can be obtained for
up to ion
masses of at least 900. Such a combination of techniques can be
invaluable in biochemical research.
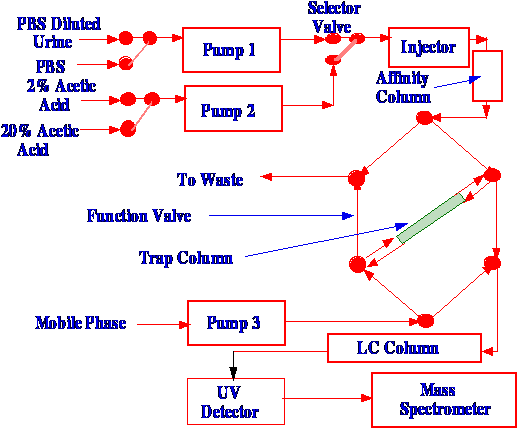
Cai and Henion [30] used a
complex
combination of sampling techniques to assay LSD and its analogs in
urine. Affinity chromatography was fist employed to extract the
substances of interest from the urine. The sample was then displaced
from the affinity column and collected in a trap, from which the
materials of interest were then displaced onto an LC column for
separation.
The
mobile phase from the column was then passed through an atmospheric
pressure ionization interface to the mass spectrometer. A diagram of
their apparatus is shown in figure 48.
Initially,
the immuno–affinity column was equilibrated with the PBS. Then
30 μl of PBS-diluted
antibody solution (10% antibody–90% PBS) was injected onto the
column. Human urine, diluted with PBS (50% urine–50 %
PBS) was then pumped through the protein G column and immediately
flushed with PBS to remove any weakly bound impurities. During this
process, the trapping was equilibrated with the mobile phase. The PBS
was then pumped through the affinity column and the trap, which
desorbed the materials from the affinity column and re-adsorbed them
on the trap. The trap was then back–flushed, and the desorbed
materials eluted through the LC column, through an API interface and
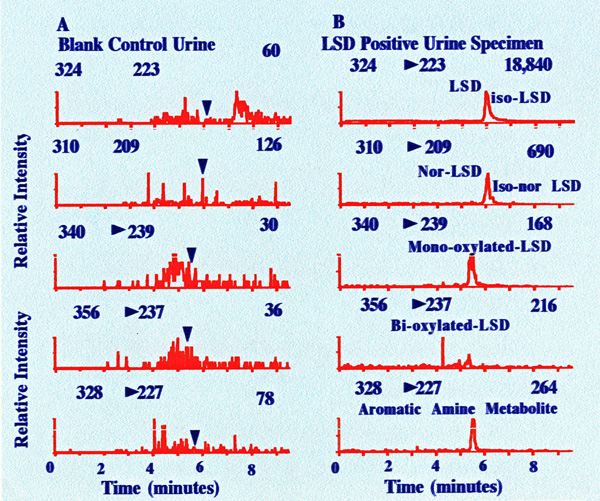
into the mass spectrometer. An example of the results obtainable by
the method is shown in figure 49. The
original concentration of LSD in the urine was 0.9 ng/ml, which shows
the very high sensitivities that can be obtained by utilizing
selective extraction by affinity chromatography. It should also be
noted that substance identification is also confirmed by the mass
Spectrum, making the procedure also useful for forensic purposes.
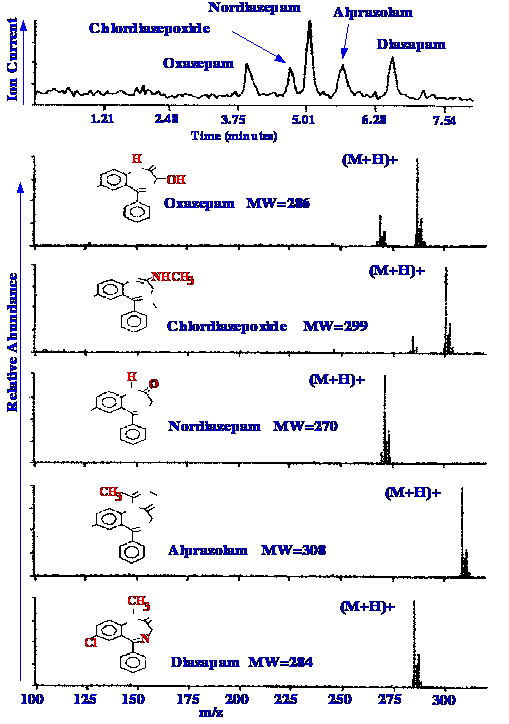
Huang et al.[31],
in their article
on atmospheric pressure ionization, demonstrated the use of the API
interface in the LC/MS analysis of some benzodiazepine s. The mass
spectrometer scanned the solution between m/z values of 100 and 350
at a scan rate of 3 s/scan.
The separation was developed
isocratically
and 25 ng of each benzodiazepine was present in the sample mixture.
The results obtained are shown in figure 50.
The top chromatogram is the
total ion
current chromatogram with background subtracted. All the
benzodiazepines were well separated and the analysis was complete in
less than seven minutes. The positive ion mass spectra obtained for
each component are shown below.
Clear and unambiguous (M+H)+
ions are obtained for each benzodiazepine with little fragmentation
of the parent ions. The lack of smaller fragments confirms the gentle
nature of the API ionizing source and offers great promise for
extended use in LC/MS analyses, and may well become more popular,
than the electrospray interfaces. Its great advantage is that it
operates at ambient pressures and temperature. In addition, the
device does not need the extensive pumping support required by the
electrospray interface.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.








