ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Electron Impact Ionization
Electron impact
ionization is
generally a fairly harsh method of ionization but the energy used to
produce the ions is controllable, which is practically an advantage.
The procedure usually produces a range of molecular fragments that in
most cases, helps to elucidate the structure of the molecule.
However, although molecular ions are often produced, which is
important for structure elucidation, sometimes only small fragments
of the molecule are observed, with no molecular ion. Under such
circumstances, alternative ionizing procedures may need to be used. A
diagram of a simple electron impact ionization source is shown in
figure 2.
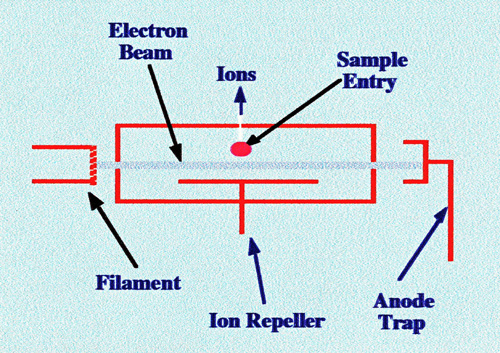
Electrons are formed by thermal
emission
from a heated tungsten or rhenium filament and accelerated by an
appropriate potential to the anode trap. The magnitude of the
accelerating potential may range from 5 to 100 V depending on the
electrode geometry and the ionization potential of the substances to
be ionized. The filament current can be automatically controlled to
provide a constant trap anode current and, thus, maintain steady
ionizing conditions. The sample is introduced into the gas stream at
the center of the electron beam. The ions formed are repelled by a
suitable potential, through a hole in the wall of the ion source
enclosure and, thus, pass into the accelerating field of the mass
spectrometer. A more
detailed and
practical layout of an electron impact source is shown in figure 3.
The central stainless steel block is made from stainless steel and
the sample is introduced through a small hole in the center.
Molecules move into the ionization chamber where they meet a stream
of energetic electrons and ions are produced. These ions are repelled
into the focus area by suitable potentials being applied to repeller
plates 1 and 2. The positive ions are accelerated by the
repeller plates and the negatively charged accelerator electrode and
pass into the analyzer unit. A magnetic field of a few hundred gauss
is often maintained along the axis of the electron beam, to confine
the electrons to a narrow helical path. In general only about 0.1% of
the molecules entering the ion source are ionized.

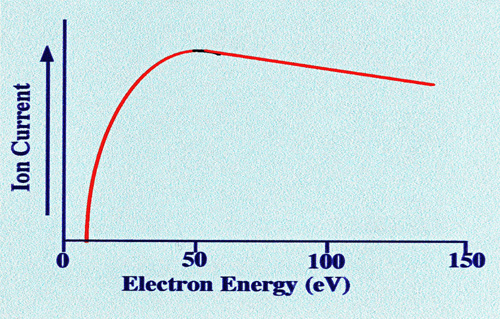
The
optimum ionization energy of the electron varies with different
compounds, but an average value appears to lie between 50 and 100 eV.
The approximate relationship between ion current and electron energy
takes the form shown in figure 4. The
ionizing energy of the electrons is controlled by the accelerating
potential applied to the anode in the ion source. This facility is
important, as it allows the energy of the electrons to be adjusted so
that optimum fragmentation will take place.
Optimum fragmentation will
provide the
maximum information to allow the structure of the compound to be
elucidated. An example of the effect of electron energy on the
fragmentation pattern of a compound is shown in figure 5. It is seen
from the pattern obtained at low energies (ca 14
electron
volts) that a large parent ion is produced but relatively few
fragments. This means that the molecular weight of the material could
be fairly easily identified but its structure would not be easily
identifiable as there were very few fragments to work with.
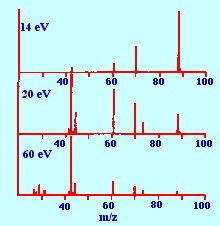
In contrast, at high electron
energies (ca
60 electron volts) there are a large number of fragments,
particularly at low molecular weight, but the parent ion is hardly
discernible. This means that although some of the secondary structure
of the molecule may be revealed, the lack of a definite parent ion
would again make the total molecular structure difficult to identify.
However, at mid–electron energies (ca 20 electron
volts)
numerous fragments together with an unambiguous parent ion are
produced, providing ample information for structural identification.
It is seen that the electron
energy in
electron impact ionization is an important parameter on which to
optimize, to ensure that the best possible data is generated for
structure elucidation. If a more gentle form of ionization is
required, however, Chemical Ionization should be used.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.






