The most common application of
ICP
ionization system is to determine element speciation. In principle,
the different compounds can be separated by liquid chromatography,
and the mass spectrometer can select those separated components that
contain the specific element or elements of interest. The IC
ionization system is applicable to this type of analysis, as it can
provide charged atoms of all the heavy elements present, and it is
the speciation of such elements that is important. The details of the
ICP torch, that is used to produce the charged atoms, has already
been described and so some examples of its use will be given.
Shum and Houk [33] employed size
exclusion
and ion exchange chromatography to separate certain metal proteins ,
and two species of selenium , SeO32-
and SeO43-.
15 pg of selenium could
be easily detected. Due to its high sensitivity and selectivity,
Atomic Spectroscopy is the preferred method for the determination of
trace elements. However, Atomic Spectroscopy cannot differentiate
between the chemical forms and oxidation states of the element, and
these can be very important, as they determine the toxicity of the
substance, and the role the element plays in biosynthesis. If a
separation technique is used to differentiate between the different
species of the element or its oxidation state, then the mass
spectrometer can unambiguously identify the peaks that contain the
element of interest. In addition, if isotope ratioing is employed, a
quantitative assay can also be accomplished.
A GPC column was used for the
separation of
the metal proteins and an ion exchange column was used for the
separation of the selenium . The mobile phase from the column passed
through a fused quartz capillary to a direct-injection nebulizer; the
distance between the inner capillary and the nebulizer tip was about
25 μm. The ICP/MS tandem
instrument was the Elan Model 250 (Perkin Elmer Sciex).
A sample of blood serum
containing metal
proteins of lead, cadmium , zinc , barium , copper, iron and sodium was
separated on the size exclusion column, and the elements monitored by
the mass spectrometer. The results obtained are shown in figure 51.
It should be noted that results for all seven metals were obtained
from a single injection. The separation of the different oxidation
states of selenium on an ion exchange column is shown in figure 52

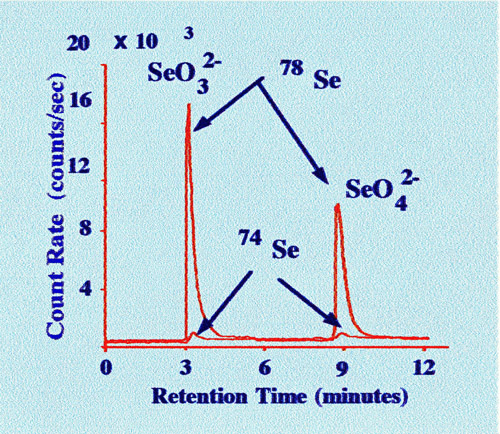
Figures
51 and 52 demonstrate the general efficacy of the system. The
detection limits for iron, copper, zinc , cadmium and lead were
reported to be 3 pg, 0.7 pg, 1 pg, 0.5 pg and 0.5 pg, respectively.
Although
the elements are unambiguously identified, the chemical nature of the
eluents can only be assessed from the chromatographic data. It would
be possible to employ an electrospray interface to another mass
spectrometer, in parallel with the ICP/Ms instrument, and this could
provide further information on the structure of the associated
protein.
Powell et al.
[34], described a
sensitive technique, employing an API interface to determine the
speciation and the quantitative assay of Cr(III) and Cr(VI). The API
interface used a direct injection nebulizer, and a sensitivity of 30,
60 and 180 ng/l was shown for the total Chromium , Cr(III) and Cr(VI)
respectively.
The sample volume was 10 μl
and, thus, the actual mass sensitivity appears to be 0.3, 0.6, and
1.8 pg of the total Chromium , Cr(III) and Cr(VI) respectively. A
diagram of the sampling arrangement is shown in figure 53.
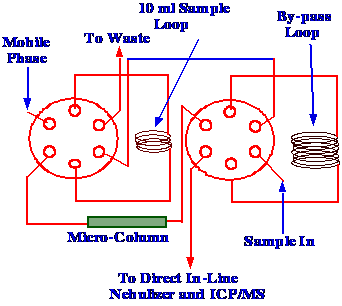
The valves were connected so
that the 10 μl
sample could either be directed through the LC column, which would
separate the different Chromium species, before the eluent passed to
the direct inlet nebulizer, or to a by-pass loop, which would allow
the total sample to be passed directly to the nebulizer.
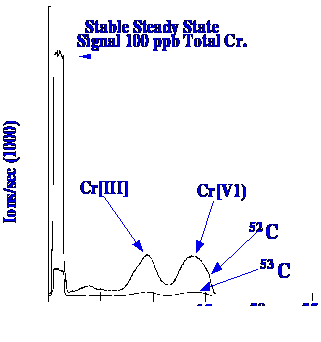
The
same procedure was used as described previously, the liquid
chromatograph separated the different species of Chromium , and the
ICP/MS identified those peaks that contained the Chromium . The two
oxidation states of Chromium were well separated and, as shown in
figure 54, both the 52 and 53 isotopes could be clearly monitored and
differentiated.dissolved in
natural
waters are important to the metabolic processes that take place in
aquatic ecosystems. Such material acts as a substrate in
heterotrophic processes (metabolic assimilation of decaying vegetable
or animal tissue) or as enzymes , vitamins or toxins in different
organisms. In natural waters, the dissolved organic material largely
comprise of humic substances. Humic substances
contain a range
of polar and dispersive functional groups and can, therefore,
interact strongly with heavy metal pollutants as well as PCBs and
PAHs and pesticides . Thus, heavy metals can be transported by the
humic substances over large distances. Alternatively, the humic
substances can trap the heavy metals and act as a detoxification
agent. It is clear that the speciation of the different heavy metals
in water can have very important ramifications. On the one hand, a
particular species can contribute to water toxicity, and on the
other, it can help in the purification of water for drinking
purposes.
Rottman
and Heumann [35] developed an apparatus to examine heavy metal
interactions with dissolved organic substances in natural waters.
They employed a LC/MS combination with an API interface and a special
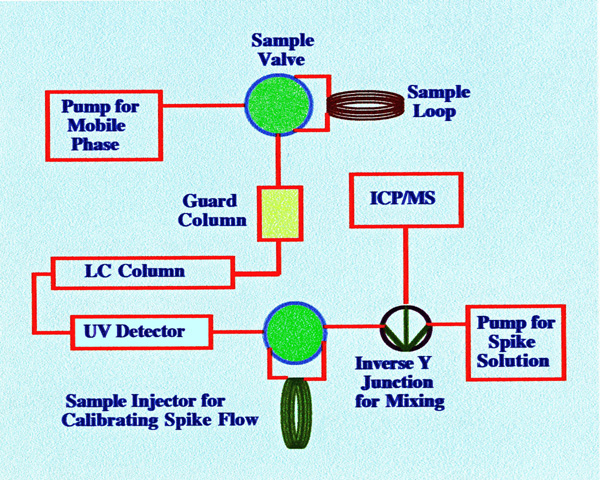
sample system. A diagram of their sampling arrangement is shown in
figure 55.The sample volume was 500 μl,
which, on injection, passed through a guard column packed with
TopOffGel 3PW, and then through a TSK 3000 PW, glass, size exclusion,
analytical column.
The
mobile phase was monitored by a UV detector and the exit flow passed
through a second sample valve (used for calibrating the spike flow)
to a Y junction where it was mixed with the spike flow. The mixture
then passed directly to the nebulizer of the ICP interface and was
then monitored by the mass spectrometer. The results obtained from
the analysis of a number of natural waters are shown in figure 56.
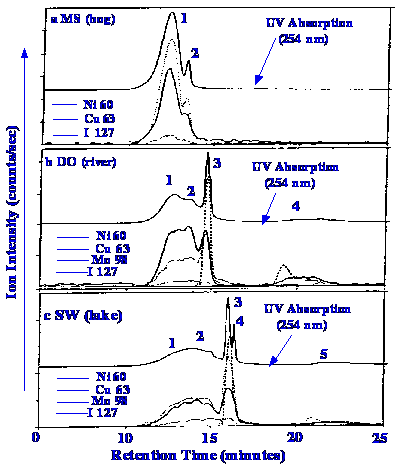
All the
chromatograms show clearly fractionated organic substances. The
components from the bog water are eluted fairly early in the
chromatogram, and as the separation was carried out on an exclusion
column, this means that the organic materials were of significantly
higher molecular weight (having larger molecular volume) than those
from the river and lake water. It is also seen that the distribution
of the different heavy metal elements, within the range of humic
compounds present, differs quite considerably between the different
water sources. The technique is obviously ideal for examining the
speciation of the heavy elements, even at the low concentrations
normally found in natural waters.
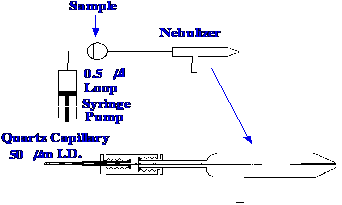
Pergantis et al.
[36] designed a
microscale system, using microbore columns and the ICP ionization
system for the detection and estimation of arsenic compounds at the
fentogram level. The system was designed to minimize
any band
dispersion that could take place between the sample valve, or column
exit, and the nebulizer. A diagram of the microscale flow injection
system is shown in figure 57.
The micro-flow nebulizer was
designed to
operate at low flow rates, i.e. in the range 10 to
150 μl/min.
The nebulizer is placed inside the ICP torch, and is designed to give
a very fine droplet spray, at the low flow rates demanded by
microbore columns.
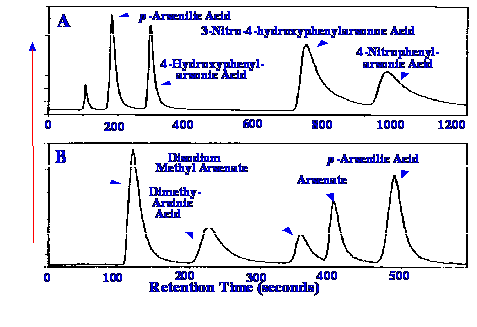
The system was used to separate
and
identify a range of arsenical animal feedstock additives, from
naturally occurring organic and inorganic arsenic compounds. The
separations obtained are shown in figure 58. The upper chromatogram
(A) shows the separation achieved by employing dispersive
interactions only, the lower chromatogram (B) was obtained by
exploiting a mixture of dispersive and ionic interactions by using an
ion pair reagent contained in the mobile phase. The lower limit of
detection was reported as 4 ng/l, which would be equivalent to 4
pg/ml. The volume of sample placed on the column is not clear, but if
it were 1 μl, then this
would be equivalent to a mass of 4 fentograms. The high resolution of
the liquid chromatograph, coupled with the high sensitivity of
ICP-MS, makes the system a very powerful tool for use in many
contemporary analytical applications.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.










