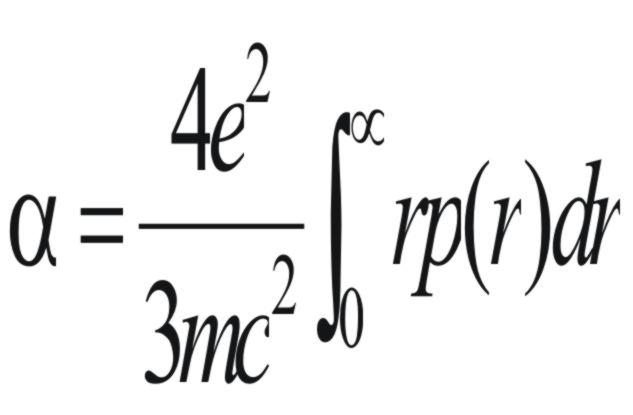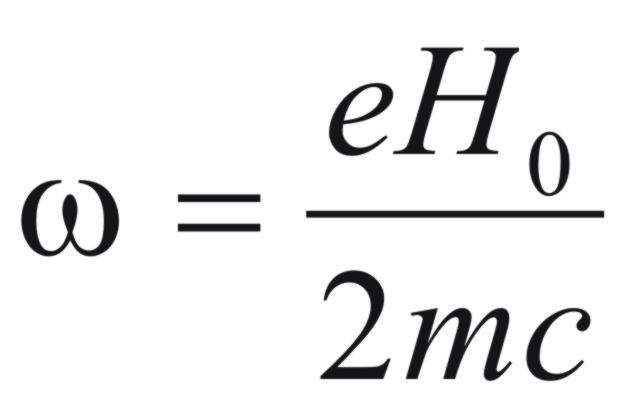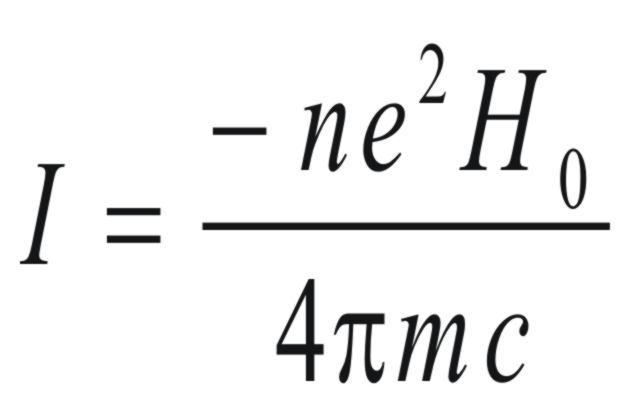Chemical Shifts
The chemical shift provides the
necessary information that permits the structure of a compound to be
identified and renders the NMR Spectrum unique to the substance
concerned. The theory that explains the chemical shift is complex and
outside the remit of this monograph. However, some of the basic
principles on which the chemical shift depends needs to be discussed
and those interested in the theoretical further details are
recommended to read the two books given in References.
The shielding effect that is
responsible for the chemical shift has already been defined by
equation (4), viz.
H= (HF+HS)(1-α) (4)
Which can be put more simply as.
H= H0(1-α)(5)
where (Ho)
is the total applied magnetic field
and (α)is
called the shielding parameter or
screening constant..
If the nucleus has no orbital or
spin angular momentum and the electrons can rotate in circles round
the direction of the applied field, (α)
can be expressed by the following equation,
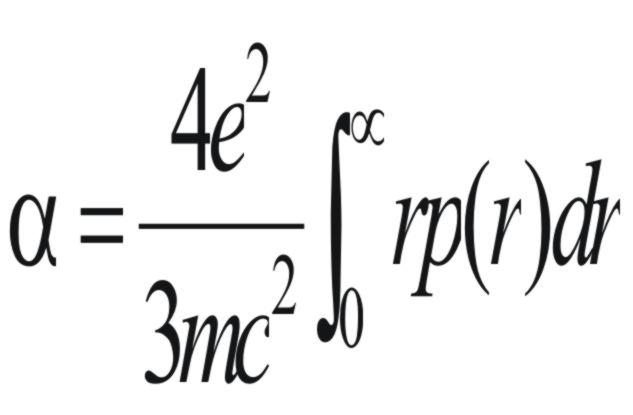
However, if attempts are made to calculate screening constants for
molecular systems the procedure becomes complicated and the screening
effects must be classified into different groups as follows.
The
diamagnetic effect of the atom. This effect is due to the
field resulting from the electron rotating round the atom.
The
paramagnetic effect for the atom. Paramagnetic shielding
resulting from the presence of close atoms.
3 Contributions
from neighbouring atoms. Shielding effects that are
transmitted from all neighbouring
atoms.
Contributions
from inter atomic currents. Currents resulting from π
electrons providing inter atomic currents
A hydrogen
atom situated in a magnetic field not only experiences the applied
field but also the field resulting from the pseudo circular current
provided by the electron rotating round the nucleus. As a result the
field experienced by the nucleus is slightly less than that from the
externally applied magnetic field (i.e.
the nucleus is shielded by the field from the rotating electron).
This is described as the diamagnetic shielding effect (see 1 above).
The diamagnetic shielding effect is depicted in figure 13.

The situation depicted in figure 13 is idealized and in the more
general case the circulation of the electron is distorted by the
presence of nearby atoms, which ’hinders’ the free
circulation of the electron and thus modifies its effect on the
magnetic field experienced by the nucleus. This additional effect is
defined as the paramagnetic effect (as given in 2 above).
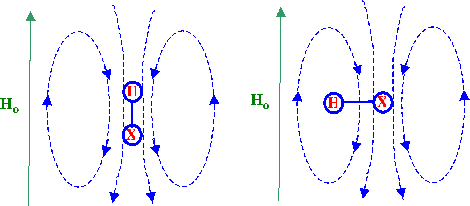
The effect of a secondary field produced by a neighbouring atom X
on a given proton is depicted in figure 14. From figure 14 it is seen
that the secondary field experienced by the proton under
circumstances where the primary field is parallel to it the primary
field will be opposed and there will be a shielding effect.
Conversely, if the primary field is perpendicular to the bond then
the net effective field will be increased and the shielding constant
will be reduced.
Consider the situation where the
acetylenic bond is lined up with the externally applied magnetic
field (i.e. a condition where the diamagnetic susceptibility
lies along the carbon-carbon bond axis. This situation is depicted in
figure 15.
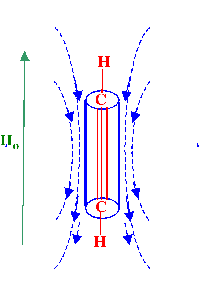
It is clear that any secondary field
resulting from anisotropy from the triple bond linkage will shield
the associated protons, In addition, it would follow that nucleus
placed perpendicular to the triple bond would cause a deshielding
effect.
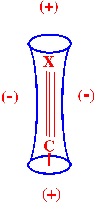
It has been generally accepted that
shielding is always denoted as (+)
whereas the process of deshielding is denoted as (-)
when denoting those areas that are associated with different
structural characteristics. This is achieved by constructing cones
around the particular structural feature and by employing the above
symbols to indicate the nature of the shielding effect. As an
example, the shielding and deshielding zones around the acetylenic
and the nitrile bond represented ‘shielding cone’ form is
depicted in figure 16.
Contributions
from inter atomic currents that result from π
electrons providing inter atomic currents are typified by the benzene
nucleus. It was suggested by Pauling that the six π
electrons of a benzene molecule would precess in a magnetic field in
a plane perpendicular to the direction of the field and the angular
frequency (ω)
would be given by,
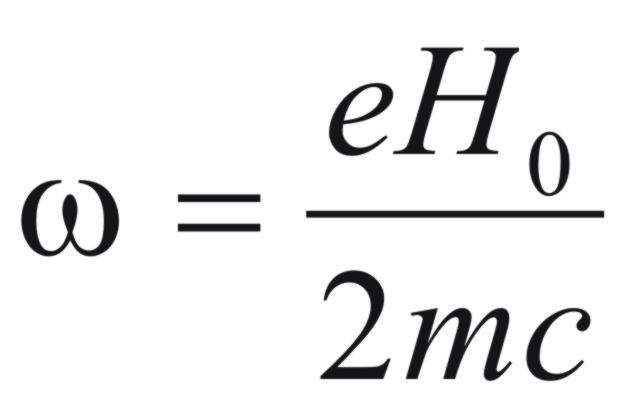
The resulting current (I) that could
be considered flowing in circle having the same radius as the benzene
ring would be given by,
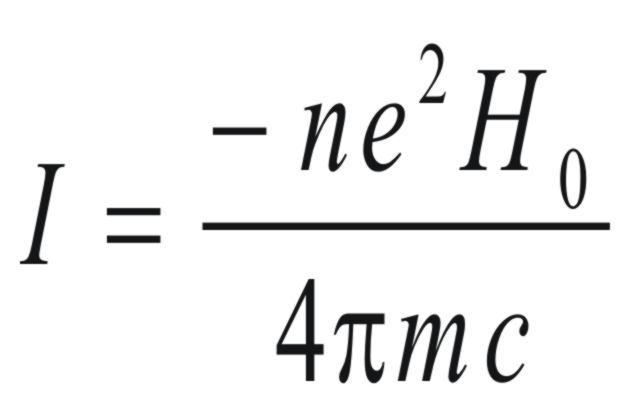
where n is the number of electrons
and e is the charge on the electron.
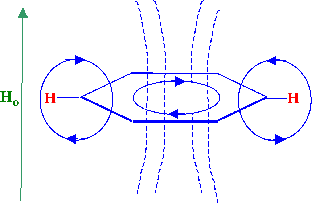
Figure 17. The Ring Current from and Aromatic Ring
The ring current from the aromatic nucleus is
depicted in figure 17 and the similarity of the system to that
depicted in figure 13 should be noted.
This discussion has only described the basic
principles involved in NMR spectroscopy and gives some indication of
the complex nature of the theory and the various different magnetic
and electromagnetic environments that are involved. The technique has
not been extensively used by analysts, or the general practicing
chemist. This is largely due to the complexity of the spectroscopic
system and the knowledge and experience necessary for the
interpretation of NMR spectra. Nevertheless, if reference spectra are
available NMR spectroscopy can be used for solute identification and
quantitative determinations. However, and for the most part, the
application of the technique to problems in chemistry needs to be
handled by trained NMR spectroscopists. In addition NMR spectroscopy
is not the most common spectroscopic technique used in analytical and
general chemistry for practical reasons; an NMR spectroscopy service
involves the use of expensive equipment, entails expensive operating
costs (general instrument maintenance and, in particular, the
maintenance of the low temperature of the superconducting magnet with
liquid nitrogen and liquid helium ) and the service of a skilled
spectroscopist.
There are many commercial laboratories that offer NMR
spectroscopy services exclusively and so, if required, it is
preferable to send samples to these service laboratories as an
alternative to tolerating the high operating costs of an ‘in
house’ NMR spectroscopy facility. In house NMR spectroscopy
services are found mostly in the research laboratories of
universities and, perhaps, a limited number of industrial research
laboratories. In such environments they are mostly employed for
research into the technique itself and for structure elucidation of
new and hitherto unknown substances and the measurement of some of
their physical properties.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.