The Absorption of UV and Visible Light
In
the practical wavelength range for UV/Visible spectroscopy (i.e.
between about 180 nm and 700 nm) the range between 180 nm and 400 nm
is considered part of the UV Spectrum and between 400 nm to 700 nm
the whole of the Visible Spectrum. The range below 180 nm is
sometimes called the vacuum
ultraviolet region.
In general, it is difficult to operate at wavelengths below 180 nm as
most substances adsorb in this region and thus, the walls of the
vessel in which the UV Light is generated will be opaque to light of
such wavelengths or transmission will be very poor. In practice, the
lower wavelength limit is, at present, about 150 nm but most UV
spectroscopic measurements made for analytical purposes are carried
out at wavelengths between 180 nm and 400 nm. The energy carried by
an electromagnetic wave is not continuous, but, as already discussed,
are propagated in finite parcels called quanta.
Radiation
is only adsorbed by a substance when the energy of the radiation
corresponds to that needed to increase the potential energy of the
substance (in some form) by one or more increments. As already stated
the transfer of energy is
largely achieved by the interaction of its electric vector with the
substance. Absorption of UV/Visible radiation changes the electronic
state of a molecule and can, for example, raise an electron from the
ground state (situated in its most stable orbit) to one of its
excited states (in some higher orbit). The electronic ground state is
one in which all of the electrons of the species are in their most
stable orbits. An electronic excited state is one in which at least
one of the electrons occupies an orbit of higher energy than that of
the ground state. It would appear that if the wavelength of the
radiation passing through a substance is gradually changed, and the
transmitted light is simultaneously monitored by an appropriate
sensor, then the curve resulting from the output of the sensor being
plotted against wavelength might show a series of sharp adsorption
lines. These lines would occur at frequencies where the radiation
energy (hν)
was equal to that of specific electronic transitions in the molecules
of the substance.
In solution, a given
molecule may exhibit numerous adsorption levels that have energies
very close to one another. The bands are so close that, in most
cases, they cannot be observed individually and, as a result, they
occur under one envelope giving a broad band in the UV adsorption
Spectrum. The breadth of the band may extend from 50 to 300 nm. This
type of absorption is shown in the Spectrum for ethyl ethanoate in
figure 4A.
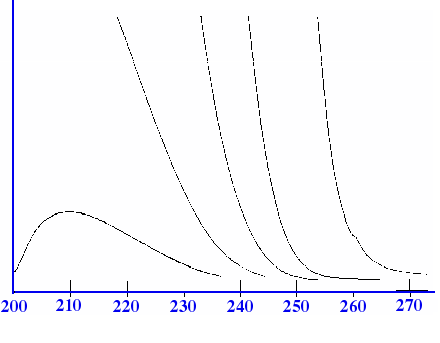
The Spectrum for
ethyl ethanoate is a very simple Spectrum containing no fine
structure and would be of little use for solute identification. In
contrast it is seen that the aromatic structure of benzene gives a
fairly complex Spectrum (shown below in figure 4B)) that could easily
be used for identification purposes. This type of spectra is typical
of aromatic compounds. Unfortunately, most compounds, particularly
those containing the ester and acid groups, give very similar spectra
to that of the ester example and, thus, can only be identified from
their UV Spectrum with considerable difficulty.
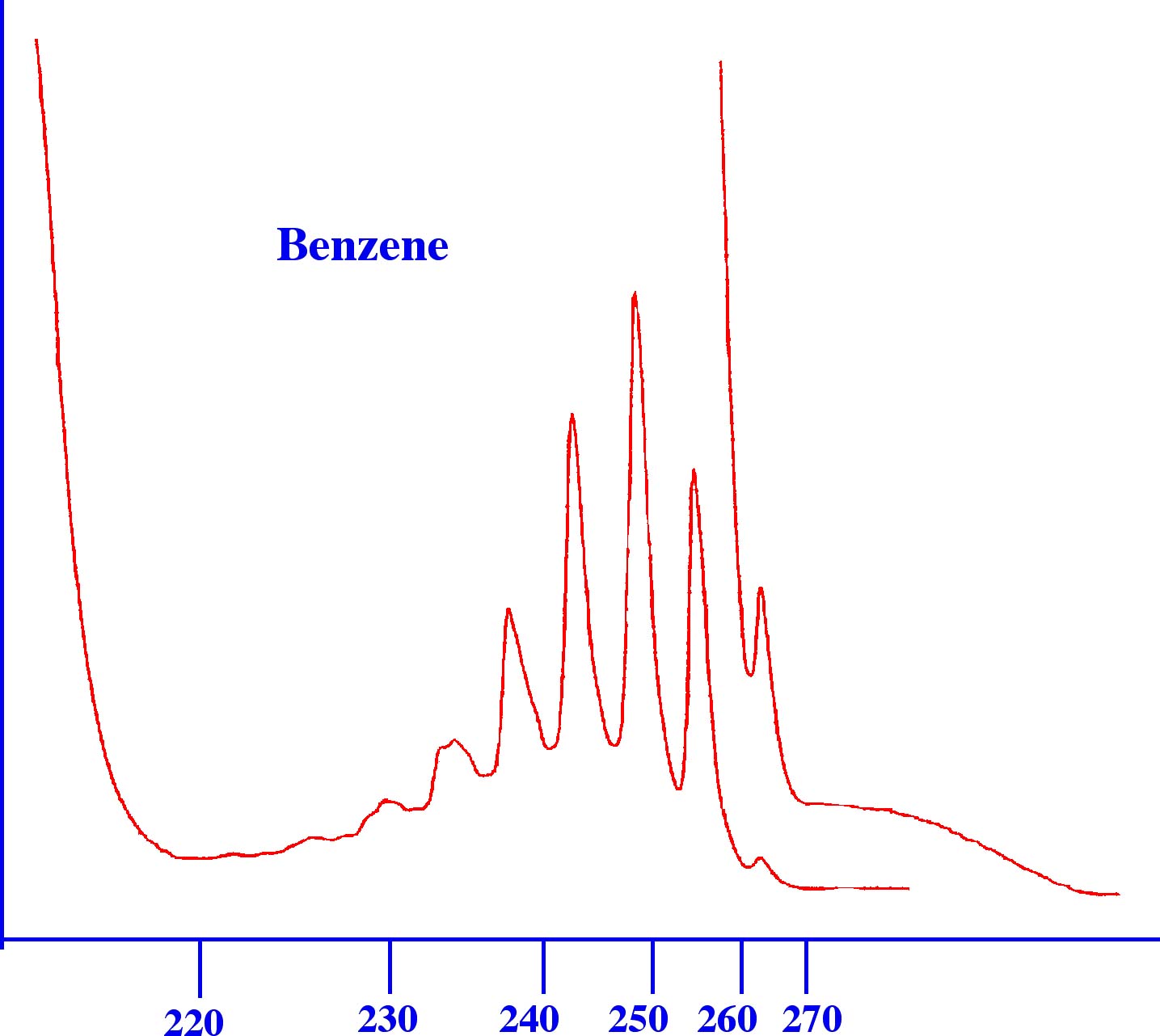
As a
consequence, UV spectroscopy is, perhaps, the least helpful of all
the spectroscopic techniques from the point of view of confirmation
of substance identity or structure identification. It is, however,
the most sensitive and the easiest to employ and, in addition, the UV
spectrometer is relatively inexpensive. Consequently, despite its
technical limitations, it is one of the more common spectroscopic
techniques to be employed in solute identification and structure
elucidation when appropriate. As the eye is often used in
colorimetric measurements, its response to Visible light needs to be
considered. A diagram representing the response of the eye to
different colours is shown in figure 5.
The
numbers on the vertical axis of the graph in figure 5 are not
absolute, only relative. It is seen that the eye is most sensitive to
green and fairly sensitive to orange and yellow. This is why most
road warning signs are now in orange and yellow as opposed to the
older signs that are in red (green is not employed as it has been
traditionally used for signs signifying safety, or permission to
pass). Police jackets and road workers jackets are in yellow and
orange so that they can be more clearly seen. It is also seen that
red and blue light have a poor retina response which makes signs with
black or blue letters on a red background (or vise
versa) difficult for
older people to see,

Although
not indicated in figure 5, the response of the retina to light
intensity is not linear so that absolute colorimetric measurements
made by visual observation may be very approximate. The eye, however,
is very sensitive to slight
changes in light intensity
and so accurate measurements can be made visually for analytical
purposes if
very similar light intensities are compared
(i.e.
by comparators, comparing the colour of a sample with that of a
standard). From the data shown in figure 5, comparative colour
measurements using green, yellow or orange derivatives would probably
give the greatest accuracy.
As
an aside, the illusion of colour is an interesting aspect of the
effect of Visible light on the sensors of the eye retina (presumably
also electric in nature) and the subsequent interpretation of the
generated signals by the brain. electromagnetic radiation of 555nm
wavelength reflected or generated by an object and striking the
retina sensor cells of the eye transmit electrical signals to the
brain that then creates the illusion of the colour green.
The object appears green in colour. However, there is no such thing
as the colour green; it is solely an illusion created by the brain
when stimulated by electromagnetic waves of 555nm wavelength. In a
similar manner, when exactly the same type of electromagnetic
radiation but having a wavelength of 650 nm stimulates the brain, the
illusion of the colour red
is produced. Again there is no such colour as red, it is merely an
illusion generated by the brain when it responds to the stimuli of
radiation having a wavelength of 650 nm. In addition, to further
complicate an already complex set of illusions,
due to the established structure of the atom, all the apparently
solid objects of the solar system (and certainly the planets and
their contents) are more than 98% empty space (not gas, just vacuum).
This will include the mountains, trees, people etc. that, although
appearing so real and genuine to human perception, are also
illusionary as they are 98% empty space and their surfaces merely
reflect light of different wavelengths (the reason for this will
become more apparent as progress is made through his book). The
combination of these illusionary concepts can be strongly thought
provoking but such thoughts are more appropriately considered in a
book on philosophy than in a book on spectroscopy.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.





