ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Permeable Membrane Interface
The membrane interface is only
suitable for
relatively volatile materials. However, as some environmental samples
are contained in aqueous matrixes (e.g. the aromatic
hydrocarbons in water, or certain pesticides in soil extracts etc.)
the present stage of the interface development will be briefly
considered for possible future use for sample solution ionization
sources. One of the reports on the design and use of membrane inlet
systems was that of Maden and Hayward, [43], who investigated a wide
range of different materials for permeability and selectivity. The
materials examined were silicone , two types of latex, polyethylene ,
polyurethane (polyether), polyurethane (polyester), a copolymer of
acrylonitrile and butadiene and polyvinyl chloride. The design of the
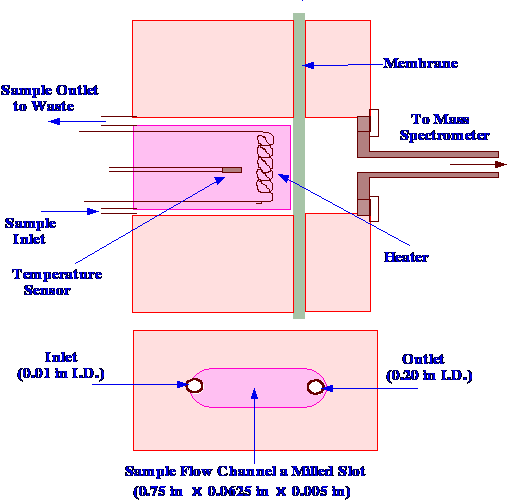
interface in which they tested the membranes is shown in figure 66.
The membrane was positioned between two stainless steel blocks, and
the entire interface was heated by a four-element heater.
The temperature controlled by
means of a
platinum resistance thermometer. The heaters were carefully located
to ensure that the whole block was kept at an even temperature. The
sample column eluent or flow injection sample, entered by a narrow
channel 0.01 in. diameter, and then passed over the membrane and out
through a larger channel, 0.02 in. diameter. The larger exit conduit
helped reduce the pressure on the membrane, and prevented mechanical
breakdown. The solutes diffused through the membrane under a
concentration gradient that was naturally set up. On the other side
of the membrane, the solute evaporated into the high vacuum of the
mass spectrometer. The vapor then passed down a heated tube into the
ion source.
The results indicated that as
one might
expect, some membranes were more efficient in the transfer of certain
types of materials that others. If the membrane was made of a polymer
that was naturally dispersive in nature (e.g.
silicone
membranes) then it would transfer dispersive type compounds such as
hydrocarbons efficiently. In contrast, the silicone membrane would
probably not be so effective for polar compounds such as methanol .
Polar membranes such as the polyurethane or polyester would be likely
transport polar materials such as alcohols more efficiently than
hydrocarbons. It would appear that the membrane interface needs some
more development work to be carried out before it might be considered
as a competitor for the electrospray or API sampling systems.
Nevertheless, it is an alternative approach, and one, which deserves
further consideration.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



