The Low Pressure
UV Discharge lamps
Today there are
many types of UV, visible, near infrared and infrared lamps available
but only a selected few pertinent to UV and Visible light will be
mentioned here. One of the early lamps used for the production of UV
light were the low-pressure mercury vapour lamps, which generate the
majority of its light, at a high intensity at 254 nm. There are a
number of other lamps of similar type that can be used to provide UV
light of a specific wavelength and they are the low-pressure cadmium
lamp which generates the majority of its light at 225 nm and the low
pressure zinc lamp that emits largely at 214 nm. None of the lamps
emits strictly monochromatic light and light of other wavelengths is
always present but usually at a significantly lower intensity. The
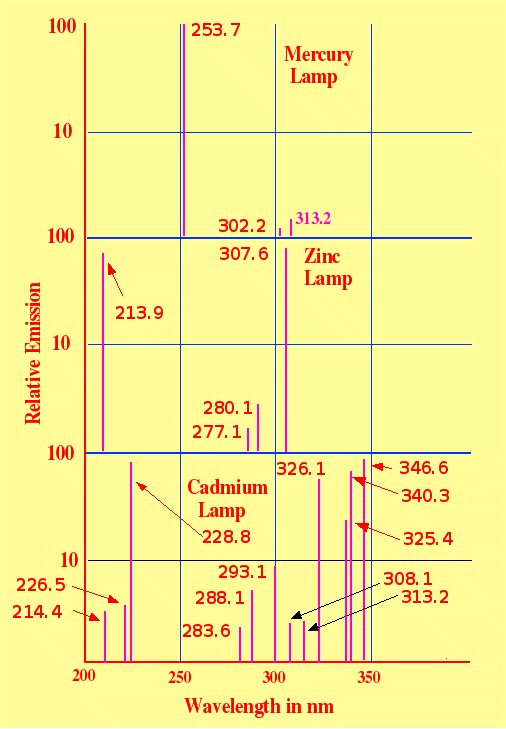
emission spectra of the mercury , cadmium and zinc lamps are shown in
figure 9
It is seen that
if a completely monochromatic source of light is required, then an
appropriate filter would be needed. The low-pressure mercury light
source (major emission at wavelength 253.7 nm) is the lamp that
provides the closest to true monochromatic light of all three lamps.
However, it does provide light of significant intensity below 200 nm,
but light of such wavelengths is often absorbed and eliminated by
walls of the discharge tube unless very pure silica is used.
The zinc lamp
has a major emission line at 213.9 but the emission line at 307.6 is
also of comparable intensity and would probably need to be removed by
a suitable filter if light of the lower wavelength was exclusively
required. The cadmium lamp has a major emission line at 228.8 but
light is emitted at both lower and higher wavelengths and so an
appropriate filter would again be desirable. Suitable interference
filters can be quite expensive to construct, which may account for
the unpopularity of these two lamps.
They do,
however, emit light at wavelengths, which would provide an increased
sensitivity to substances such as proteins and peptide s, which might
make their use worthwhile in the biotechnology field.
The deuterium
Lamp
The deuterium
lamp, in one of its many forms, is probably the most popular
broadband UV emission lamps. A photograph of a deuterium light source
and a deuterium lamp is shown in figure 10.
Deuterium Light Source
Deuteriun
lamp
Courtesy of “Newport Resource
and Spectra-Physics
Stray light can
be a difficult problem in UV spectroscopy as many UV Light sources
have a black body Spectrum with low UV and high Visible output. As a
result, the intensity of the Visible light often exceeds the
intensity of the real signal of the UV. However, with deuterium lamps
the intense continuum up to 400 nm and the low Visible and infrared
emission gives a high signal to noise ratio for the UV measurements.
As a result, deuterium lamps are the preferred source for UV
spectroscopy. mercury lamps can give an improved performance at
specific wavelengths but for obvious reasons, they are not suitable
for continuous Spectrum work. deuterium lamps have been constructed
with a range of modifications to meet different specifications. For
example the case of the lamp can be made of impure silica , which
restricts the emission of the lower wavelengths (lower limit about
200 nm) and, thus, eliminates the production of ozone . Other lamps
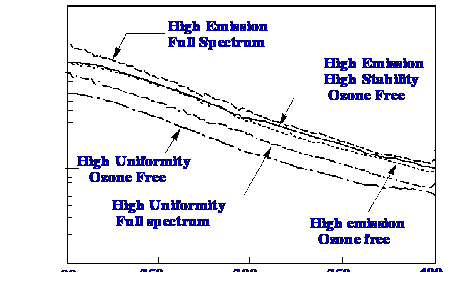
are designed to provide maximum emission others maximum stability. An
example of the emission curves for a range of different deuterium
lamps is shown in figure 11.
Courtesy of “Newport Resource and
Spectra-Physics
Xenon Flash
Lamps
Xenon lamps are
exceedingly strong light sources and are widely employed in many
photometric instruments. Flash lamps can have certain advantages over
constant current DC operated lamps as they exhibit a relatively
higher UV emission when operated in small pulses. Power supplies for
such lamps must provide both high voltages and high currents and also
a carefully controlled voltage pulse to strike the arc and, thus,
initiate the discharge. The lamp is made of pure silica containing
xenon or krypton or a mixture of both and contains three hermetically
sealed electrodes. The lamp may be straight, U shaped, circular or
spiral.
The anode is
usually made of tungsten and the cathode of porous tungsten filled
with a barium compound to reduce the work function of the electrode
and, thus, increase its electron emission. Whereas DC operated lamps
have pointed electrodes to keep heat away from the quartz envelope,
flash tubes usually have cathodes with flat discharge surfaces Two
electrodes carry the arc current and the other initiates the arc. The
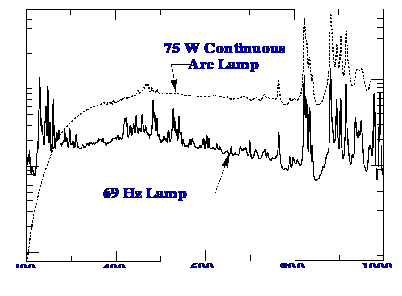
emission characteristics of Xenon lamps operated in the continuous
and pulsed mode are shown in figure 12. It is seen that the pulsed
lamp provides far more light at the low UV wavelengths than does the
continuously operated lamp.
The pulsed Xenon
lamp is frequently used as the excitation light source for the
Fluorescence spectrometer. The discharge tubes used for the Xenon
flash lamps often have carefully arranged guiding electrodes that
ensure that the electric discharge has repeatable arc paths and,
thus, ensures that a stable light output is achieved. Light stability
can be maintained to within 2%.
Courtesy of “Newport
Resource and Spectra-Physics
The Tungsten
Halogen Lamp
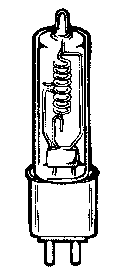
The tungsten
halogen lamps are the most popular Visible and near infra red light
sources due to their smooth spectral emission curve and that they do
not exhibit peak outputs at specific wavelengths as do other light
sources. A diagram of a tungsten halogen emission bulb is shown in
figure 13.
 Courtesy
of “Newport Resource and Spectra-Physics
Courtesy
of “Newport Resource and Spectra-Physics
The
filaments of most tungsten lamps are doped and the whole is contained
in a quartz envelope. The envelope
is filled with a rare gas containing a trace of a suitable halogen
gas. The tungsten filament operates at about 3000K. Without doubt the
Tungsten Halogen Lamp is the ‘work-horse’ of those light
emitters used for Visible and near infrared spectroscopy.
The relative
existence of the tungsten filament, a grey body radiator and a full
radiator is shown in figure 14. It is seen that the tungsten surface
is about equivalent to the grey body radiator and about 40% of the
ideal full radiator. Tungsten is not the only metal that can be used
for Visible and near infra red light emission but it appears to be
the metal of choice for most spectrometer manufacturers. From the
curve in figure 14 it is seen that the wavelength of the light
emitted from the Tungsten Halogen Lamp comprehensively covers not
only the Visible range of wavelengths but also the near infrared
range as well.

Courtesy
of “Newport Resource and Spectra-Physics
The Lamda
UV/Vis/NIR Spectrometers (950, 850 and 650)
The Lamda series
of instruments are typical of those available for UV/vis/NIR
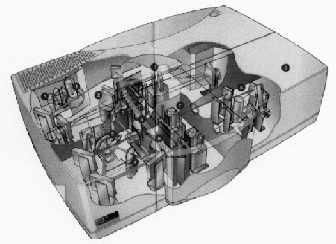
spectroscopy applications. A diagram of the general layout of a Lamda
instrument is shown in figure 15. This particular instrument was
designed to accommodate a wide variety of sample types, including a
range of sampling modules that simply clip into place as required.
The sample and detector compartments can also be rapidly changed to
suit the nature of the sample. The instrument can be fitted with
deuterium and tungsten/halogen light sources and, to ensure low stray
light, a double holographic grating monochromator is used. A common
beam mask allows a precise adjustment of beam height to match samples
of different dimensions and a common beam depolarizer permits the
accurate measurement of bi-refringent samples. A chopper switches
between sample and reference beam, and to accommodate highly
absorbing samples, sample and reference beam attenuators are
provided. A high sensitive photomultiplier and Peltier-controlled
lead sulphide sensors are available for detection. A special
universal reflectance accessory is also available that allows the
angle of measurement to be changed with no adjustment to the sample
or optics.
Courtesy of
the Perkin Elmer Corporation
1.Deuterium an
Tungsten/Halogen Light Source.
2. Double
Holographic Grating3. Common Beam
mask. 4.Common Beam Deploarizer. 5.Chopper.6. Sample and
Reference Beam attenuators. 7. Sample compartment.8.
Photomultiplier. 9. Second sampling area.
The Perkin Elmer
Lamda 25/35/45 series of UV/vis spectrometers are simpler and
accurate alternatives but with no near infrared (NIR) facilities. The
double beam system, that allows references to be measured in real
time, provides high stability, and the sealed quartz coated optics
ensures consistent lifetime performance. The spectrometers employ
deuterium and tungsten lamps and fast scanning facilities are
provided.
The results of
some standard tests carried out on the instrument are shown in
figures 16A and 16B.
The
test utilizes a 0.02% w/v toluene in n-hexane
with a 1nm slit width. The ratio of the peak and trough near 269 nm
and 266 nm respectively was found to be greater than 1.9 the
pass criterion being at least 1.5. In the wave length accuracy test
using a 4mg/ml solution of holmium perchlorate a summary of the
results are as follows.
Specified
Value (nm) Measured Value (nm) Maximum Tolerance (nm)
241.15
241.10
+/-1.0 361.50
361.10
+/-1.0 536.30
536.40
+//3.0
It is seen the
results are well within the required criteria.
16A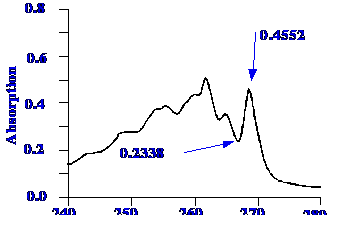
16B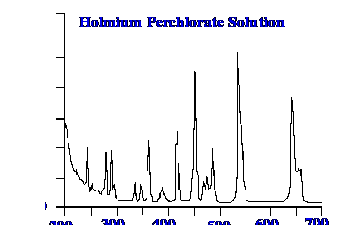
Courtesy
of the Perkin Elmer Corporation
Matrix Spectrometer
The Orial matrix
spectrometer incorporates an interesting device that allows very weak
signals in the 190-500 nm range to be measured with a resolution of
0.6 nm.
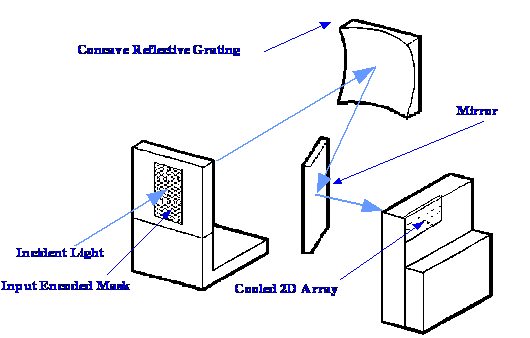
Courtesy of Newport Resource and Spectra-Physics
This
device is ideally suited for Fluorescence applications, thin film
reflectance and atomic emission. A photograph of the instrument is
shown in figure 17 and the optical layout in figure in figure 18. The
capability of an optical system to transmit radiation is measured by
the optical
throughput or the
Etendu.
For any given optical system the etendue (E)
is constant and is defined as the product of the entrance aperture
(or slit area) and the solid angle through which the light is
accepted.
Consider an
input aperture that consists of an optical matrix containing N x N
apertures.
Now
if (Ω)
is the solid input angle,
(f)
is a factor that takes into account that only a fraction of the
aperture
elements
are transparent,
(g)
is a factor that takes into account that a number of pixels are
discarded
for
one row of the mask
Then the
etendue is given by E = f x g x N2
x Ω
For the
systems being considered, f = 0.5, g = 3 and
n = 24
Thus, for a slit the same
height as the mask, E = 3 x 24 x 1 x Ω
For a fibre input
(24 μm in diameter) E = 1
x 1 x Ω
For the Matrix
Mask E = 0.5 x 3 x
N x N x Ω
= 864 Ω
It is seen that
the etendue of the mask matrix is 12 time greater than a slit and 864
times greater than a single fibre.
Courtesy of
Newport Resource and Spectra-Physics
The device has
been given the (perhaps a little vainglorious) name Multimodal
Multiplex Spectroscopy (MMS). Essentially, it consists of a 2.3mm x
0.58 mm encoded aperture mask, a concave grating, a cooled
two-dimensional Diode Array and a compute data processing system.
The
cooled aperture consists of 2304(28 x 32) lithographically etched
apertures having dimensions 24 mm x 24 mm. The total perforated mask
dimensions are 2.3 mm x 0.58 mm. Light enters the optical system
through a mask providing a total of 2304 virtually point light
sources. The etched mask must be fully illuminated by the source. A
fixed concave grating is employed having a groove density of 200
lines per mm. The peak efficiency of the grating is at 300 nm. The
sensor can be cooled to below 20oC
to provide a good signal to noise ratio and takes the form of a back
thinned 512 x 512 CCD (charged couple device) array (a sensor similar
to that employed in a digital camera). Each pixel accepts light from
2304 point light sources. The dark current output is automatically
taken before the measurements are started, the integration period is
then set and the data collected. The software can process emission
transmission, reflectance and adsorption data. The advantages of the
etendue differences calculated above are demonstrated in figure 19. A
slit/fibre spectrometer maps each spectral channel separately onto a
pixel column in the two dimensional detector. However in the
Multimodal Multiplex system each pixel in the detector measures
several spectral channels simultaneously which results in a
significant improvement in the signal-to-noise ratio. The improvement
in signal to noise is clearly demonstrated by the results shown in
figure 19.
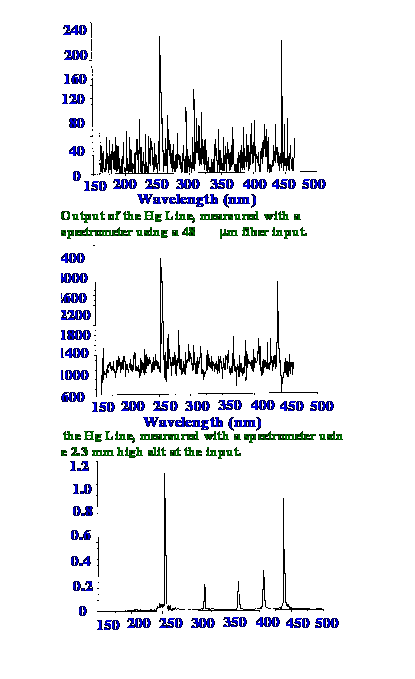
Courtesy of
ìNewport Resource and Spectra-Physics
The
emission of a mercury lamp
was measured employing three different configurations each with an
integration time of 4 seconds. The top Spectrum represents the
results from a 48 μm
fibre input, the middle Spectrum from a 24 μm
slit and the lower Spectrum from the
MMS system. The results
demonstrate the large improvement in the signal-to-noise ratio that
is achieved by the Multimodal Multiplex system. The quantum
efficiency of the system is shown in figure 20.
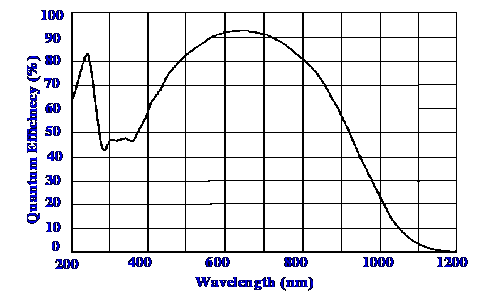
Courtesy of “Newport
Resource and Spectra-Physics

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.






 Courtesy
of “Newport Resource and Spectra-Physics
Courtesy
of “Newport Resource and Spectra-Physics







