
Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
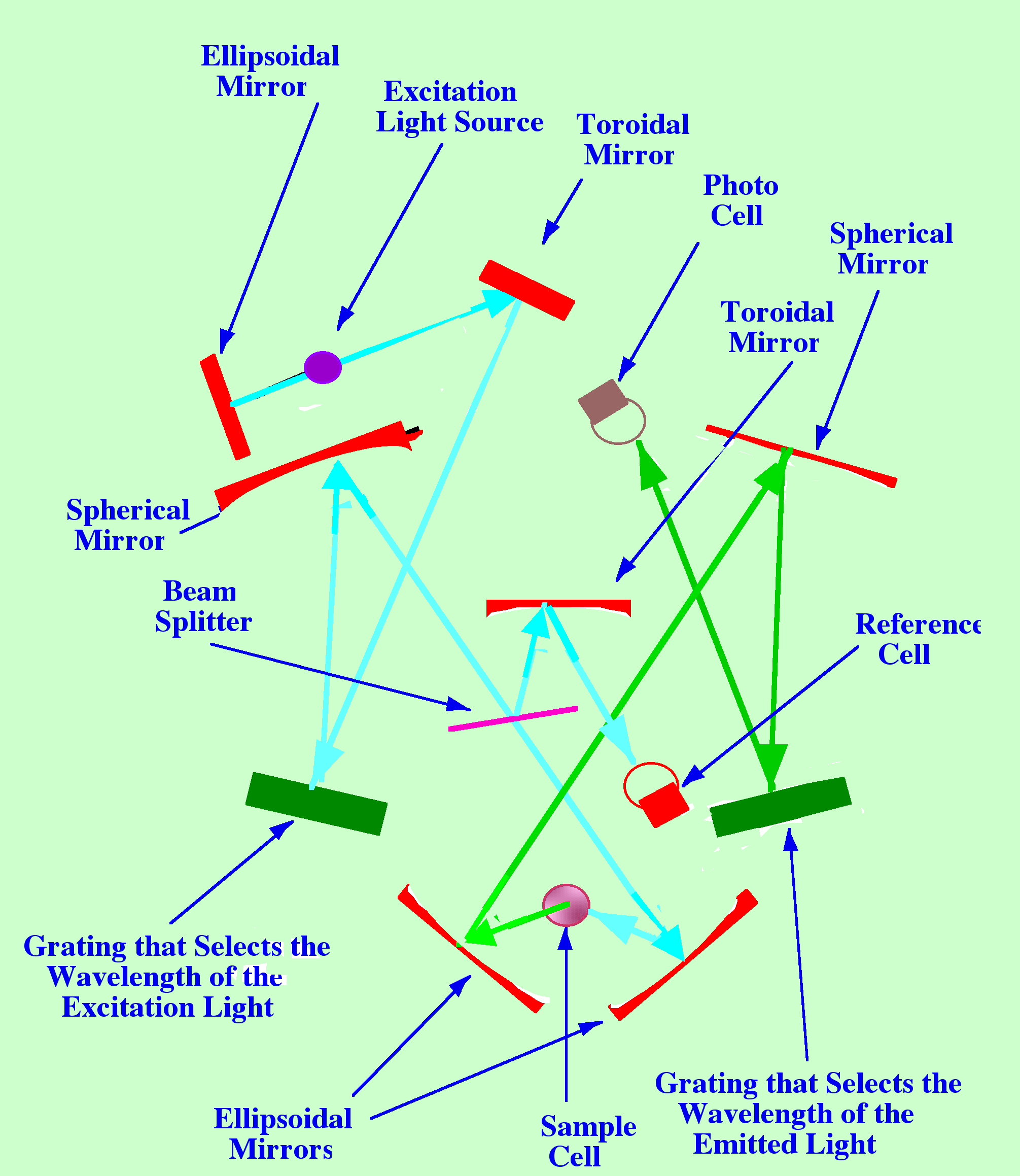
Figure
8 shows the layout of the basic Fluorescence spectrometer.
Courtesy
of the Perkin Elmer Corporation
The
excitation source (a deuterium or Xenon lamp) that emits UV Light
over a wide range of wavelengths is situated at the focal point of an
ellipsoidal mirror. This mirror is shown at the top left hand corner
of the diagram. The consequent parallel beam of light falls onto a
toroidal mirror that focuses it onto a Diffraction Grating. This is
shown on the left-hand side of the diagram. Rotation of the grating
allows the frequency (or wavelength) of the excitation light to be
selected, or the whole Spectrum scanned which would provide an
excitation spectra.
Light
of the selected wavelength then passes first to a spherical mirror
and then to an ellipsoidal mirror (shown at the base of the diagram)
that focuses the light onto the sample. Between the spherical mirror
and the ellipsoidal mirror, (in the centre of the diagram,) is a beam
splitter that reflects a portion of the incident light onto another
toroidal mirror. This toroidal mirror focuses the portion of incident
light onto the reference photocell providing an output that is
proportional to the strength of the incident light.
Fluorescent
light from the cell is focused by an ellipsoidal mirror onto a
spherical mirror (at the top right-hand side of the diagram). This
spherical mirror focuses the fluorescent light onto a grating
(situated at about the centre- right of the figure). This grating can
select a specific wavelength of the fluorescent light to monitor, or
scan the fluorescent light and provide an emission fluorescent
Spectrum. Light from the grating passes to another photocell, which
monitors its intensity. The instrument is rather complex and, as a
result, rather expensive.
However,
from the point of view of measuring Fluorescence spectra it is
extremely versatile. Much less sample is required to produce useful
Fluorescence spectra compared to UV spectra and, in general,
Fluorescence spectra contain more fine detail and are, thus, more
useful for solute identification where sample size is limited. It is
seen that as both the wavelength of the excitation light and that of
the fluorescent light can be exclusively selected, so the spectra are
not liable to same errors as those of from the Diode Array as already
discussed in the book on UV/viz spectroscopy.
The
Fluorescence spectrometer can be used in a unique way in conjunction
with the liquid chromatograph by programming the excitation
wavelength and emission wavelength to provide the maximum sensitivity
for each component of the mixture as it is eluted from the column.
Thus, once the separation has been developed and retention times
identified, the spectrometer is programmed to monitor each peak at
the optimum excitation and Fluorescence wavelengths. This procedure
provides the ultimate in sensitivity when using Fluorescence
detection.
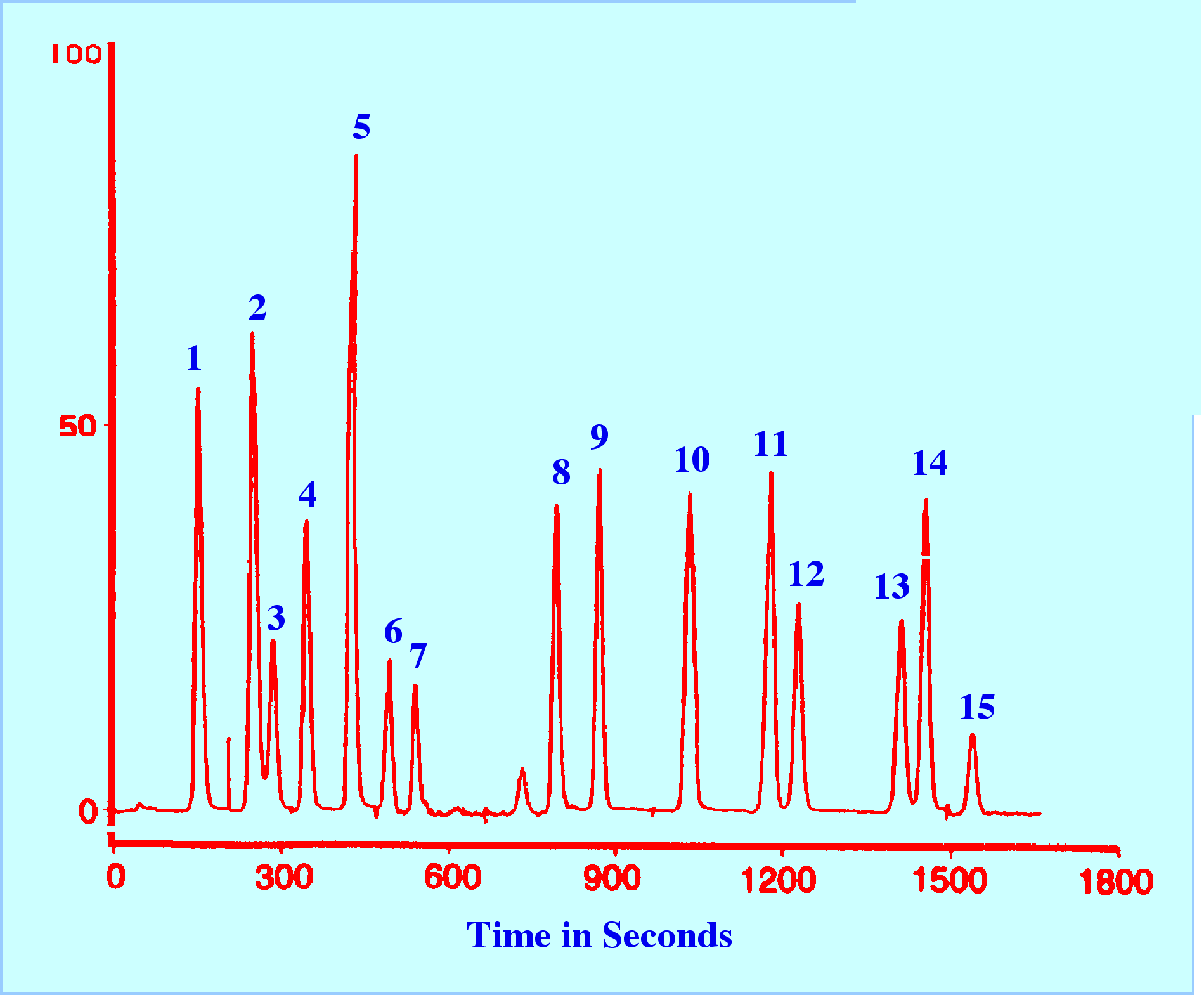
The
principle of optimizing both the excitation and emission light
wavelengths to obtain maximum sensitivity, however, can become quite
complex as shown by the separation of some priority pollutants
carried out on an early PE LC/FL tandem instrument and depicted in
figure 9. The separation was carried out on a column 25 cm long, 4.6
mm in diameter, and packed with a C18 reversed phase. The mobile
phase was programmed from a 93% acetonitrile, 7% water mixture to 99%
acetonitrile, and 1% water mixture over a period of 30 minutes. The
gradient was linear and the flow rate was 1.3 ml/min.
Table
2 The Fluorescence Excitation and emission Program
| Time - seconds |
wavelength of Excitation light |
wavelength of emitted light |
| 0 |
280 nm |
340 nm |
| 220 |
290 nm |
320 nm |
| 340 |
250 nm |
385 nm |
| 510 |
260 nm |
385 nm |
| 720 |
265 nm |
420 nm |
| 1050 |
290 nm |
430 nm |
| 1620 |
300 nm |
500 nm |
|
1
Naphthalene
|
9
Chrysene
|
|
2
Acenaphthene
|
10
Benzo(b)fluoranthene
|
|
3
Fluorene
|
11
Benzo(k)fluoranthene
|
|
4
Phenanthrene
|
12
Benzo(a)pyrene
|
|
5
Anthracene
|
13
Dibenzo(a,h)anthracene
|
|
6
Fluoranthene
|
14
Benzo(ghi)perylene
|
|
7
Pyrene
|
15
Indeno(123-cd)pyrene
|
|
8
Benz(a)anthracene
|
|
All
the solutes are separated and the compounds, numbering from the left,
are given in the figure 9. The separation illustrates the clever use
of wavelength programming to obtain the maximum sensitivity. The
program is shown in Table 2.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.




