
Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
If
examined with a polarimeter, some lines of the scattered radiation
(not all) may be polarized and, furthermore, they will be polarized
to different extents. The reason for this is not immediately obvious
and can be best explained using the following simple case of Raman
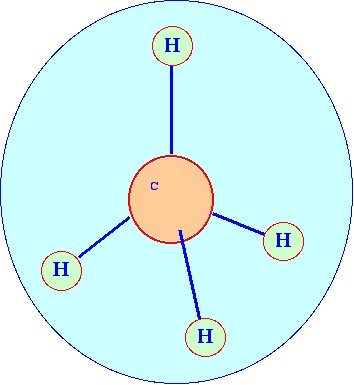
scattering. Consider a molecule, the polarizable ellipsoid of which
is spherical (typified by the molecule of methane ) which is depicted
in figure 13. The spherical
form of a
polarizable ellipsoid is given the term spherical
top.
There
are various forms of vibration that methane can take and as the
polarizable ellipsoid, in this case is spherical,
one form of the vibration (known as the symmetric
stretch) is where
all four C-H
bond distances increase and decrease in phase. Thus, the ellipsoid
shape remains spherical (for obvious reasons the frequency of these
vibrations is sometimes referred to as the ‘breathing
frequency’)
and the molecule in this form will plainly be Raman active throughout
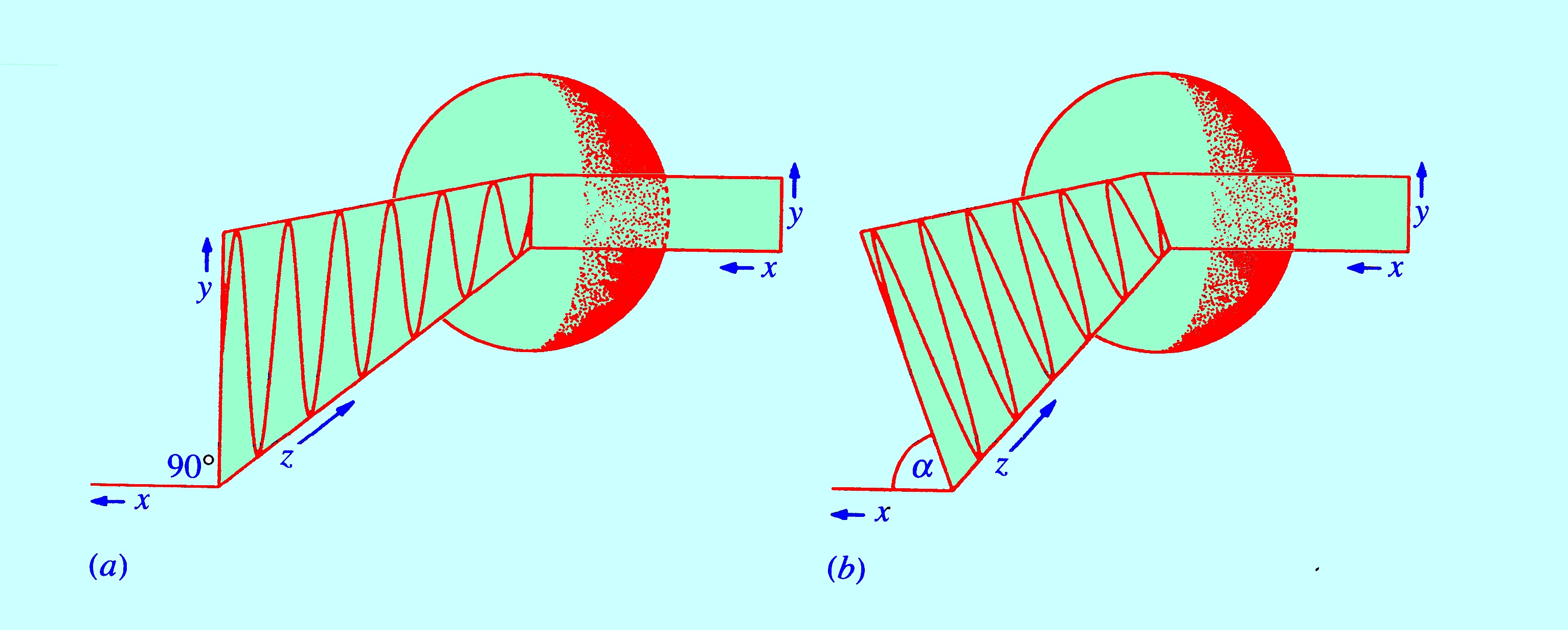
the vibration. Consider a beam of unpolarized radiation travelling
in the direction of the (x)
axis falling on the molecule. Since the polarizable ellipsoid is a
sphere it follows that it is equally polarizable in all directions
and any induced dipole will be along the greatest electric vector of
the radiation (i.e.
along the (xy)
plane). This situation is depicted in figure 14. In figure 14, two
incident beams are shown striking the molecule but it should be noted
that induced dipole is in
the (xy)
plane for both. A non-polarized beam will contain components having
all possible values of (α)
As
the induced dipole lies in the (xy) plane the oscillating dipole will
emit radiation that is plane polarized. Thus, for the breathing
frequency the Raman line will be completely polarized. For any other
type of vibration the polarization ellipsoid will not be spherical
and the axis will be randomly oriented and, thus, the scattered
radiation will no longer be polarized. This phenomenon can be
observed and measured employing the apparatus shown in figure 15.
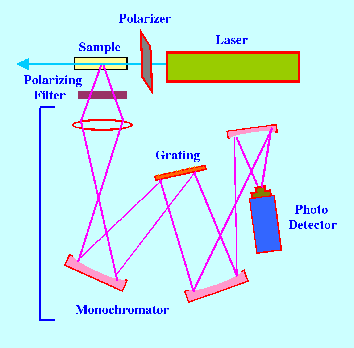
The
apparatus is very similar to the Raman instrument previously
described except that a polarizing unit is placed in line with the
laser radiation to produce light polarized in the plane of the paper.
In addition a polarizing filter is placed in line with the scattered
light that can pass either light polarized in the plane of the paper
or set at right angles and only accept light that is not polarized in
the plane of the paper. As a consequence,
in the first setting the total strength of the radiation polarized in
the plane of the paper plus
all other scattered light (IT)
will be measured. In the
second setting only scattered light that is not polarized in the
plane of the paper (IS)
will be measured. Thus, taking the previous example, the proportion
of scattered light that arises form the breathing frequency (α)
will be given by,
α = IS/IT
and this will be a
measure of the degree of polarization of that line. This principal of
identifying and measuring certain types of energy can be extended to
other substances and those interested and requiring further
information the books listed in the references are recommended.
Raman techniques are
recommended over infrared techniques for vibrational measurements as
the scattered light occurs in the UV and Visible region.
Consequently, simple construction materials such as quartz and glass
can be used and the more difficult materials such as sodium chloride
and other IR transparent materials are avoided. In addition water
strongly absorbs in the IR whereas water only produce weak Raman
scattering and so aqueous solutions can be easily examined. As result
Raman Spectroscopy lends it self to the examination of biological
materials.
An example of the
application of Raman Spectroscopy is given by the Spectrum of
myoglobin depicted in figures 14 and 15.
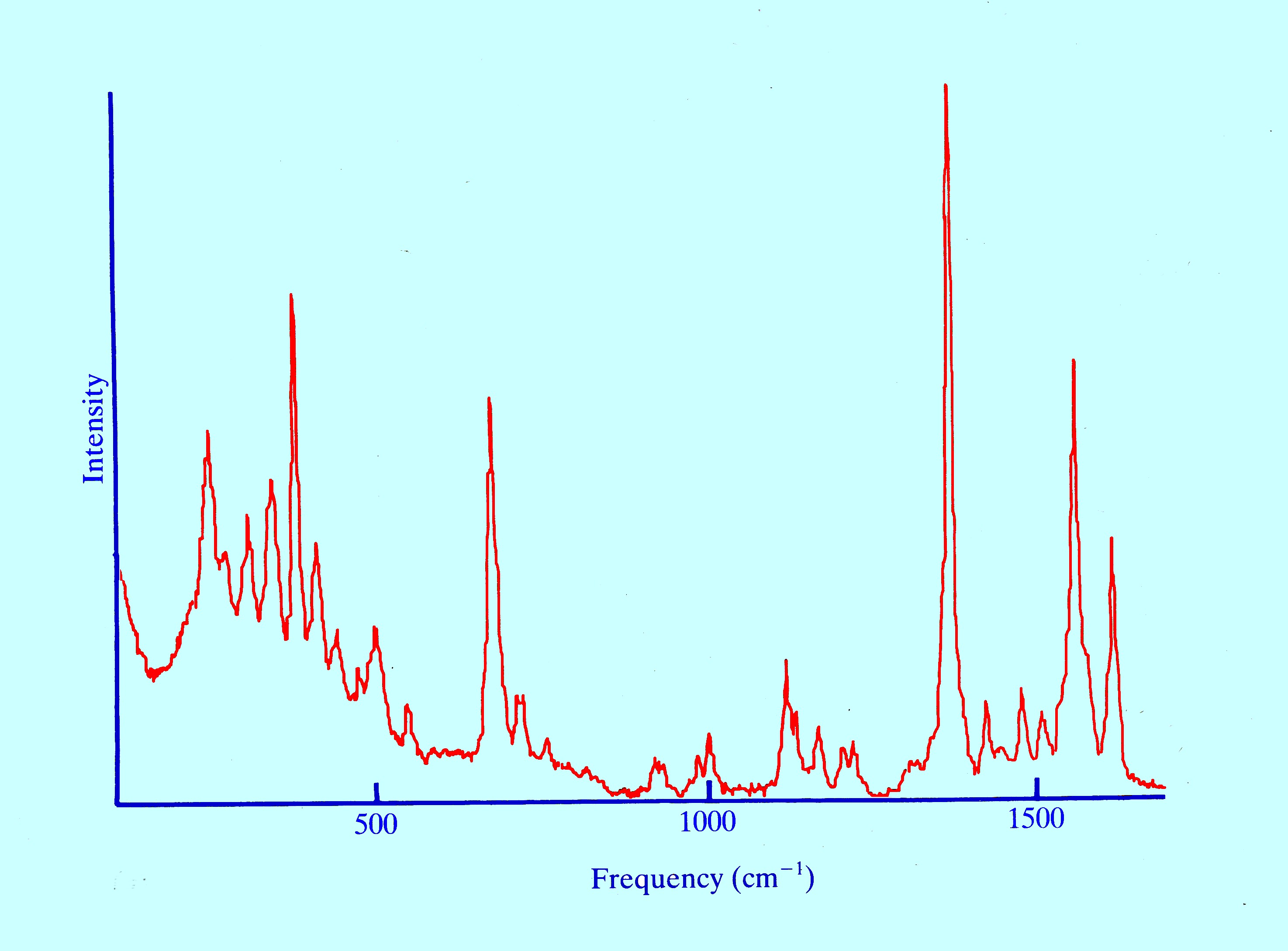
There
are a number of Raman modes exhibited arising mostly from the protein
chains. myoglobin contains iron that is capable of reversibly bonding
oxygen and carbon dioxide but more strongly carbon monoxide.
Consequently myobloblin can carry gasses around the body for
biosynthetic purposes and carry waste gases away. It follows that
myoglobin is essential in natural muscle function. It is also
responsible for the toxic
properties of carbon monoxide. Details
of the iron-carbon stretching region for myoglobin
bound to carbon monoxide (between 400 and 560 cm-1)
are shown in figure 15.
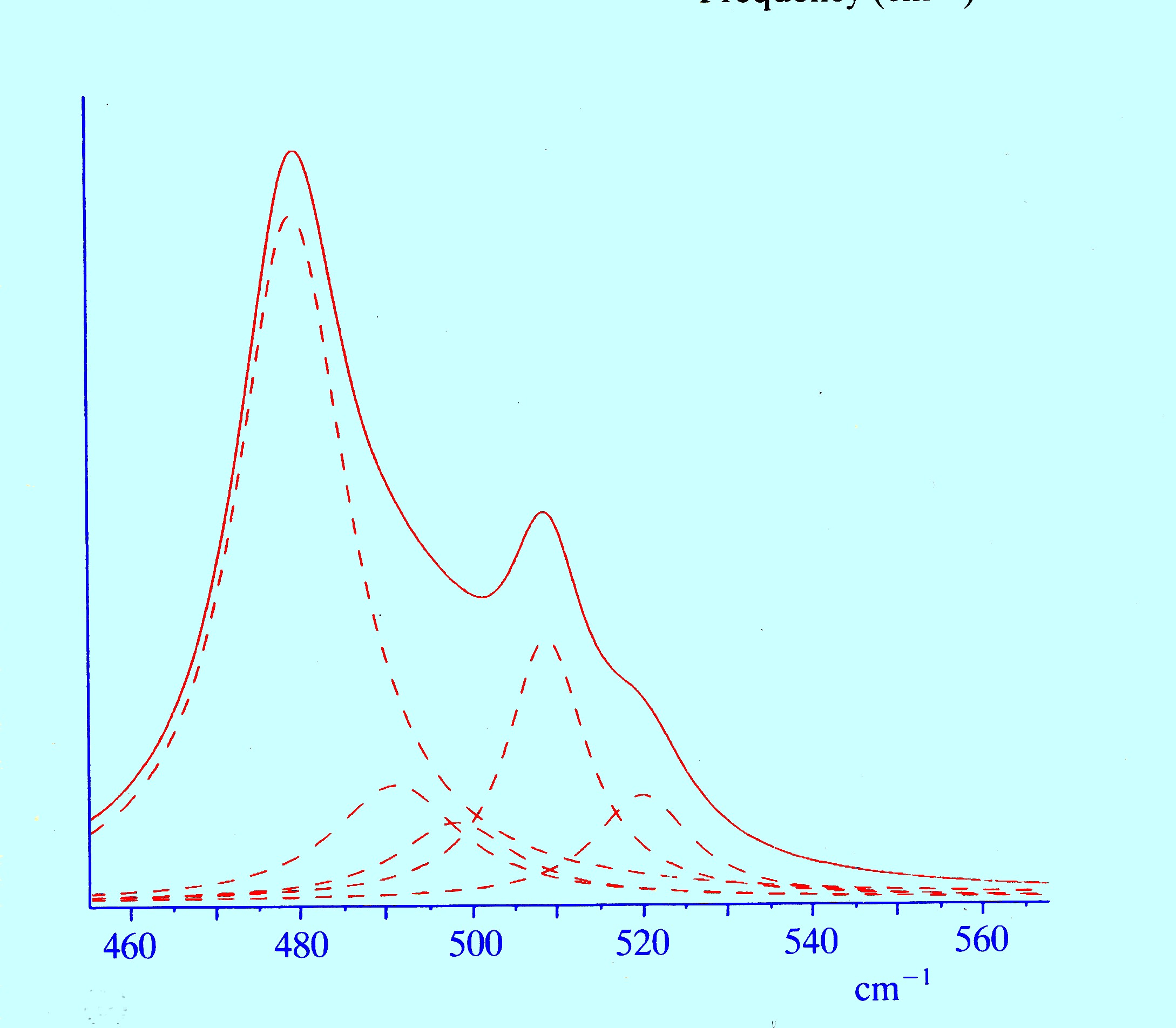
The
dotted curves are the result of the computer deconvolution of the
main envelope and exposes the presence of several peaks which
indicate that carbon monoxide may exist in different forms in
different myoglobin molecules.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.







