
A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Introduction to Spectroscopy
Spectroscopy
is the study of the absorption and emission of electromagnetic
radiation by matter. The electromagnetic radiation of analytical
interest ranges from γ
rays that have a frequency of about 3x1018
and wavelength of about 10 pm, to the radio waves used in NMR having
a frequency of about of 3x106
and a wavelength of about10 m. This range encompasses UV and visible
spectroscopy, Fluorescence spectroscopy, infrared spectroscopy,
Raman Spectroscopy, ESR (electron spin resonance spectroscopy) and
NMR (nuclear magnetic resonance spectroscopy). There is another very
important, so- called analytical spectroscopic technique, called mass
spectroscopy (MS), which is somewhat an anomaly, as it does not deal
with the adsorption or emission of electromagnetic radiation but the
separation of ions of different masses. In its early development, the
resolution of different ion masses was included as a spectroscopic
technique largely because the curves relating ion intensity to ion
mass bore some graphical similarities to true electromagnetic wave
absorption spectra.
An
electromagnetic wave consists of a sinusoidal electrostatic field
acting at right angles to, and in phase with, a sinusoidal magnetic
field. A diagram of an electromagnetic wave is shown in figure 1.
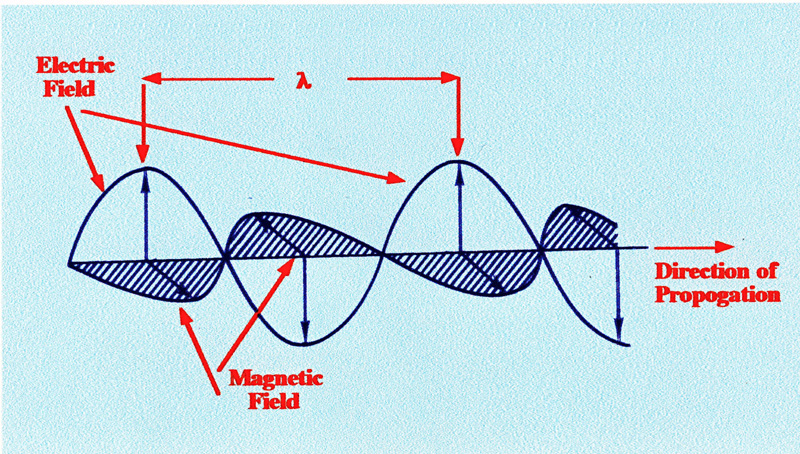
It
is the electric vector that has the major effect on matter; it is the
electric vector that activates the cells in the retina of the eye and
provides sight; it is the same vector that activates the light
sensitive surface of a photoelectric cell, which then responds to the
intensity of light falling on it. All electromagnetic radiation
travels at the same velocity (c) viz., 2.997925
x 108
ms-1
which can be approximated to 3
x 108
m/s.
electromagnetic radiation has two characteristics, its frequency and
its wavelength which are related by the following equation,
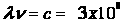 (1)
(1)
where
((λ) is the
wavelength of the electromagnetic wave, and
((ν) is the frequency
of the electromagnetic wave.
The
relationship between wavelength and
frequency for the different regions is shown in figure 2.
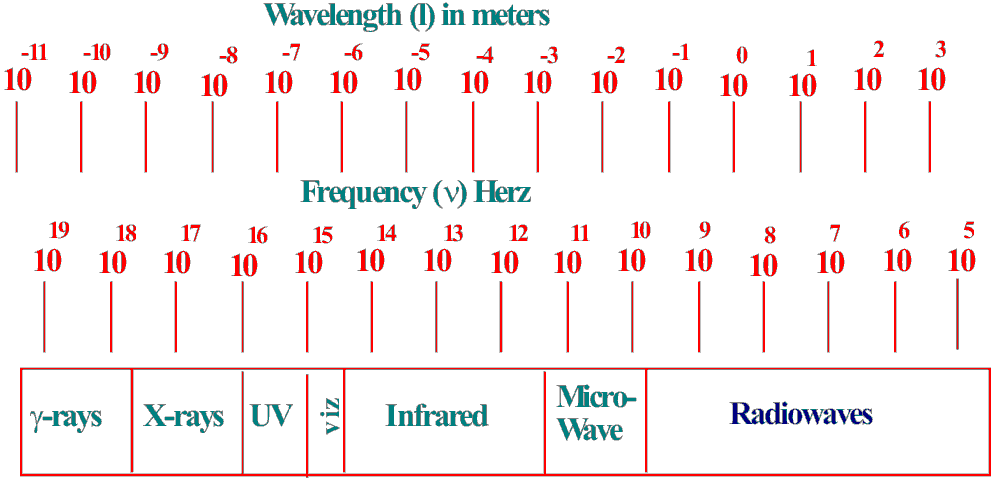
The regions
given in figure 2 does not help the analytical chemist much as it
does not indicate how the different frequencies or wavelengths are
related to the physical chemical processes with which they are
associated. This relationship can be explained as follows.
γ Rays-These
rays, that have frequencies lying between ca
3x1018
and 3x1020
Herz (100pm-1pm wavelength), are capable of exciting atomic nuclei. γ
Ray spectroscopy is not normally used in general analytical work and
the energies involved amount to 109-1011 joules per gram atom.
X-Rays - These
rays, having frequencies lying between ca
3x1016
and 3x1018
Herz (10nm-100pm wavelength), can excite inner electronic transitions
in an atom and are used extensively in X-ray crystallography. Inner
electronic transitions involve energies that may be as great as ten
thousand joules per gram atom
Ultra Violet and
Visible Radiation -
Ultra
violet and Visible radiation, having frequencies lying between ca
3x1014
and 3x1016
Herz (1μ-10nm
wavelength), can excite outer electronic vibrations (valence
electrons) in atoms and absorption or emission of light of this
wavelength range is commonly used in analytical techniques to
identify aromatic compounds, olefins and substances having one or
more double bonds and
unshared electrons. The excitation of a valence electron involves the
movement of electronic charges in the molecule from one energy level
to another. The energies involved in such transitions amount to some
hundreds of kilojoules per mole. Absorption of radiation in this
frequency range is also used in certain types of sensing devices that
are employed in chromatographic detectors.
Infrared and
Microwave Radiation - Infrared
and part of the microwave band, having frequencies lying between
ca
3x1010
and 3x1014
Herz (100μm-1μm
wavelength), can excite molecular vibrations and rotations.
Absorption of electromagnetic radiation in this wavelength range can
be used to confirm the identity of specific compounds and to identify
explicit chemical groups
in a molecular structure.
Short Wave Radio
Radiation -Short
wave radio radiation, having frequencies lying between ca
3x108
and 3x1010
Herz is employed in electron spin resonance studies.
Medium Wave
Radio Radiation -
Medium
wave radio radiation, having frequencies lying between ca
3x106
and 3x108
Herz is used in nuclear magnetic resonance studies.
Einstein,
Planck and Bohr suggested that electromagnetic radiation could also
be considered as a stream of particles in discrete energy packets
called quanta and the
energy (E) of each particle of frequency (n)
was given by,
E = hν (2)
where (h)
is Planck’s Constant = 6.62 x 10-34 Js = 6.62
x10-27
ergs/sec
Using equation
(2), the energy associated with any transition whether electronic,
rotational or vibrational, induced by radiation of a specific
frequency can be calculated.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.




