Sampling Procedures
Irrespective
of the procedure used for sampling, the device needs to be
constructed of materials that are transparent to IR light of the
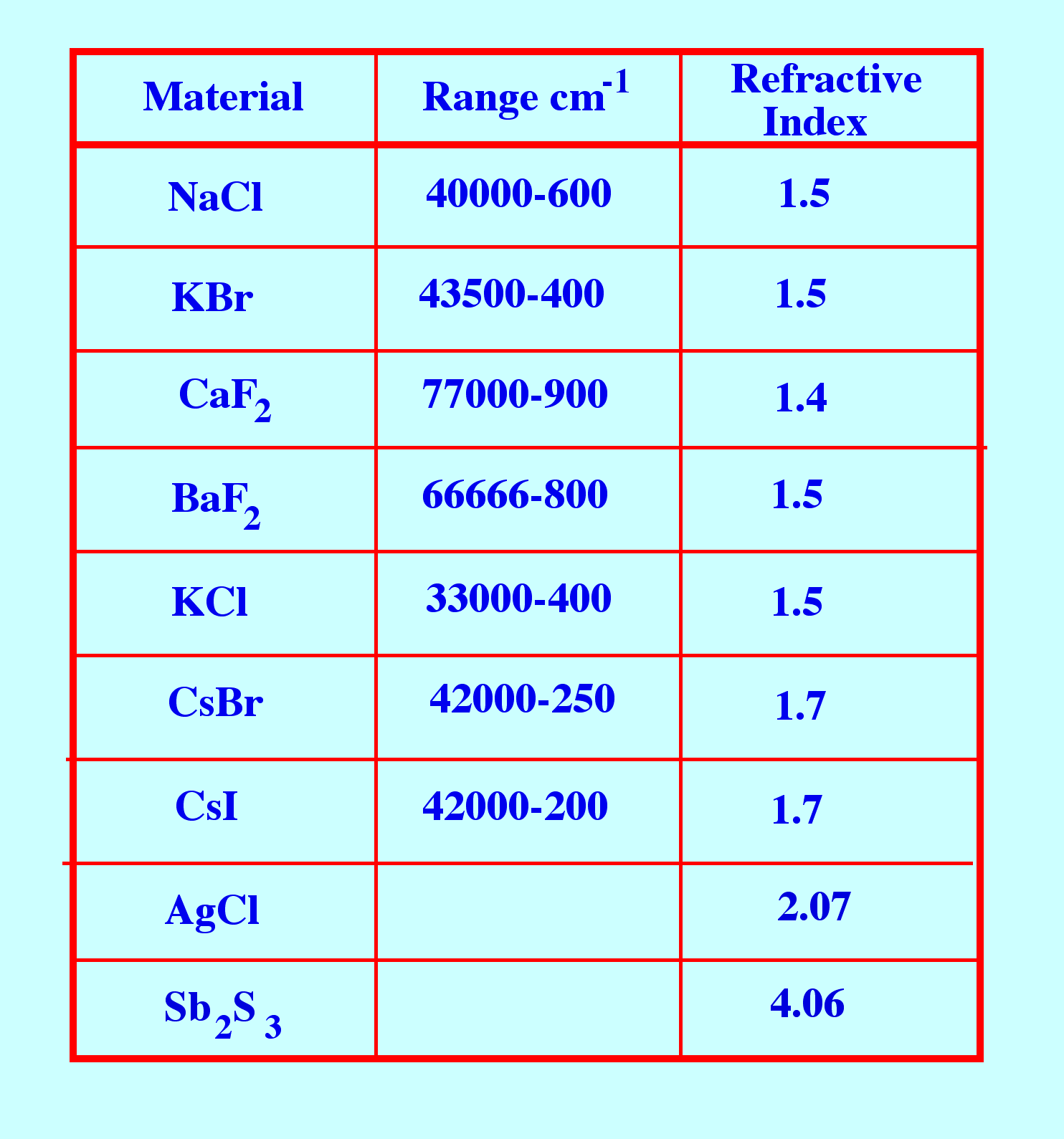
appropriate wavelength range. A list of those substances that can be
used as windows for infrared sample cells is given table 2.
Sodium
chloride is soluble in water and slightly soluble in alcohol so these
solvents cannot be used to dissolve the sample. Mechanically sodium
chloride is fairly strong and can be easily polished. potassium
bromide is similar to sodium chloride but is hygroscopic and also
slightly soluble in ether . Calcium fluoride, is insoluble in water
and is resistant to many acids and bases and does not fog. It is
mechanically strong and, thus, can be used at elevated pressures.
Barium chloride is thermally and mechanically susceptible, insoluble
in water but soluble in acids and ammonium chloride.
Table
2. Some Materials that are Transparent to Infrared Light
Potassium
chloride, having similar properties to sodium chloride, is less
soluble but hygroscopic.
Cesium
bromide is hygroscopic and soluble in water and acids.
Cesium
iodide is hygroscopic and soluble in water and alcohol.
A
simple infrared cell can consist of two gaskets on either side of
each window that are separated appropriately to provide the required
sample volume between them. The sample can be dissolved in an
appropriate solvent or, alternatively, the sample may be spread
between two IR transparent plates pressed together so the sample
exists as a thin film. This technique can only be satisfactorily
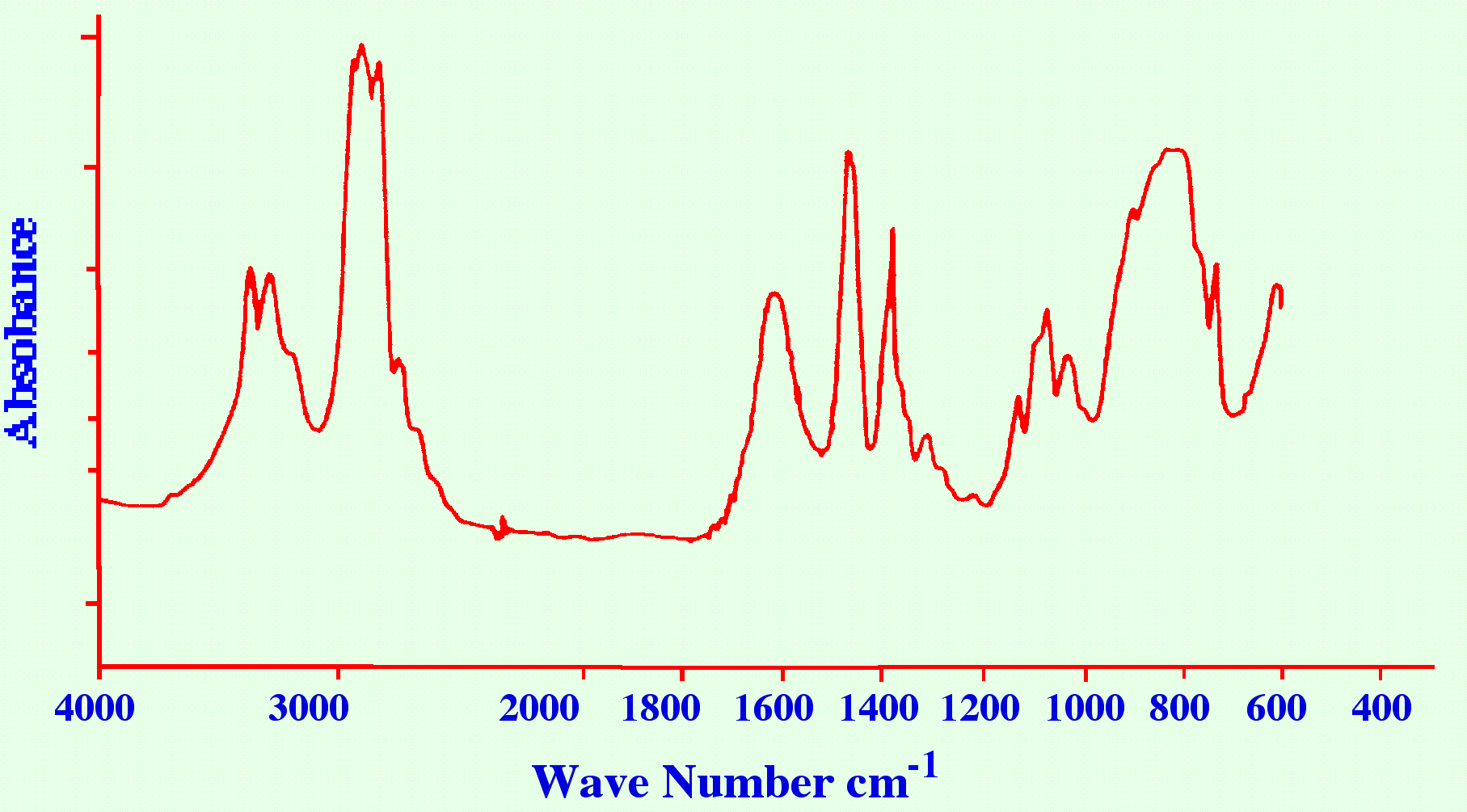
employed if the sample is relatively involatile. A Spectrum obtained
in this manner for hexylamine is shown in figure 12.
This
sampling procedure can be very rapid. If a solvent is used, it must
be chosen so that the sample is adequately soluble and should be
non-polar and have limited groups that will provide IR absorption or
interact with the sample. In most cases the Spectrum of the solvent
must be subtracted from the Spectrum of the sample solution to obtain
the Spectrum of the sample alone. There are three methods of
obtaining spectra from solid samples and they are, dispersion in
pressed Halide Disks, dispersion in mulls and as films.
Halide
Disks Samples
To
obtain a sample dispersed in a halide disk, a few milligrams of the
sample is mixed with about 150 mg of dry halide powder and ground in
a mortar. The mixture is then pressed under vacuum into a disk in an
IR disk press.
The pressure employed is usually about 1.6 x 105 kgcm-2. At this pressure the material is sintered and a clear transparent
disk should be produced. The most commonly used alkali halide
employed for this purpose is potassium bromide, which, as shown in
table 2, is transparent in the mid-infra red region.
Mull
Samples
To
prepare a mull, the sample is first ground to a fine powder in a
pestle and mortar and then about 50 mg is suspended in about 20 μl
of a mulling agent. The suspension is further ground into a smooth
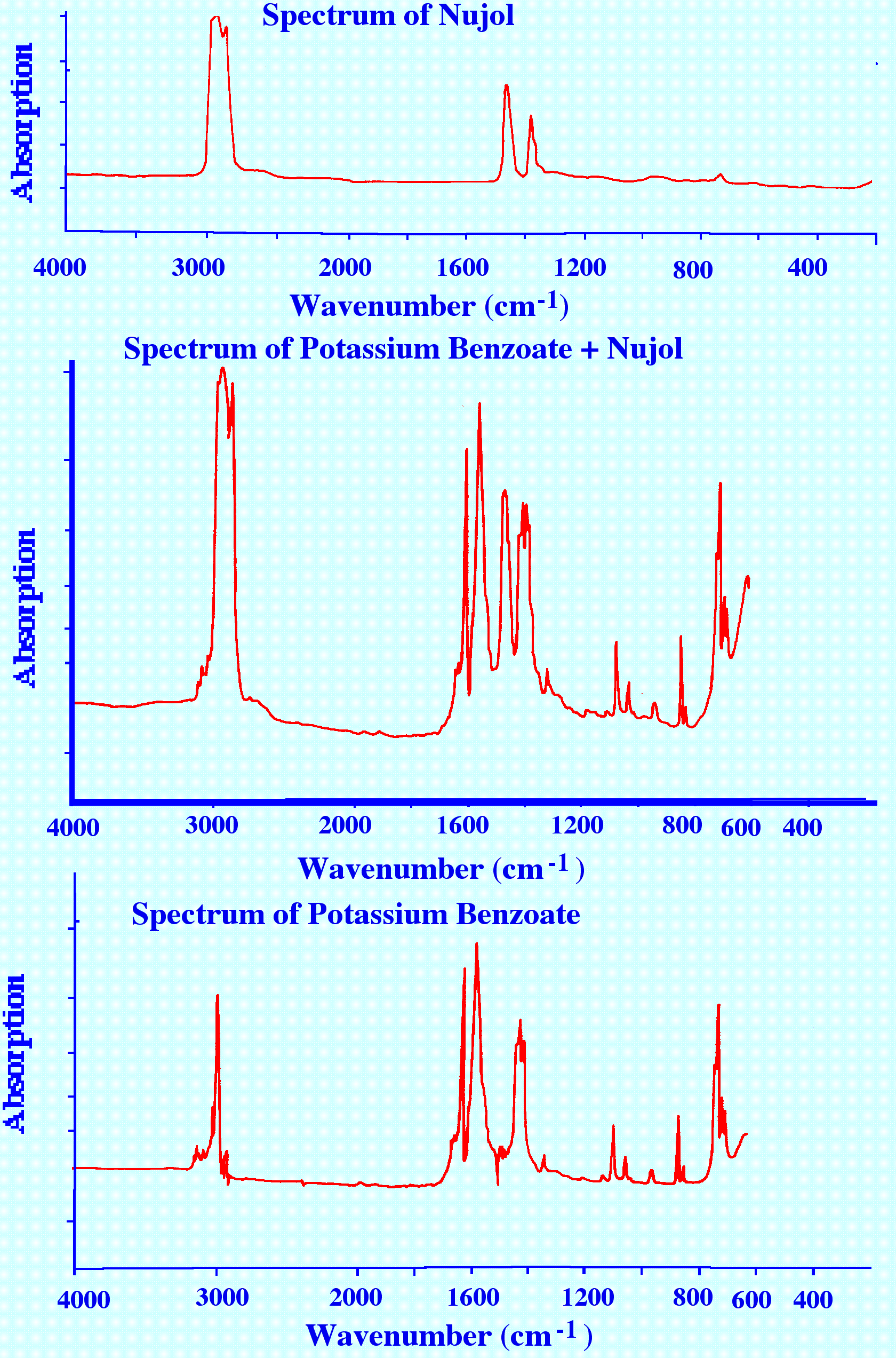
paste. One of the more common mulling agents is liquid paraffin sold
under the name of Nujol . To obtain suitable spectra the proportions
of sample to Nujol may need to be adjusted and the particle size of
the sample must be sufficiently small. An example of a the Spectrum
of potassium benzoate obtained by subtracting the Spectrum of Nujol
from the Spectrum of potassium benzoate plus Nujol is shown in figure
13.
Film
Samples
Film
samples are particularly useful for the examination of polymers . They
can be either formed by deposition from a solvent or by melting the
samples and pressing between two plates. The film is then pealed from
the plates and supported appropriately in the infrared light path.
The film can also be formed on an infrared window using a similar
procedure. If the sample melts at an appropriate temperature and
remains stable, it can be hot-pressed by means of a hydraulic press.
Vapour
and Gas Samples
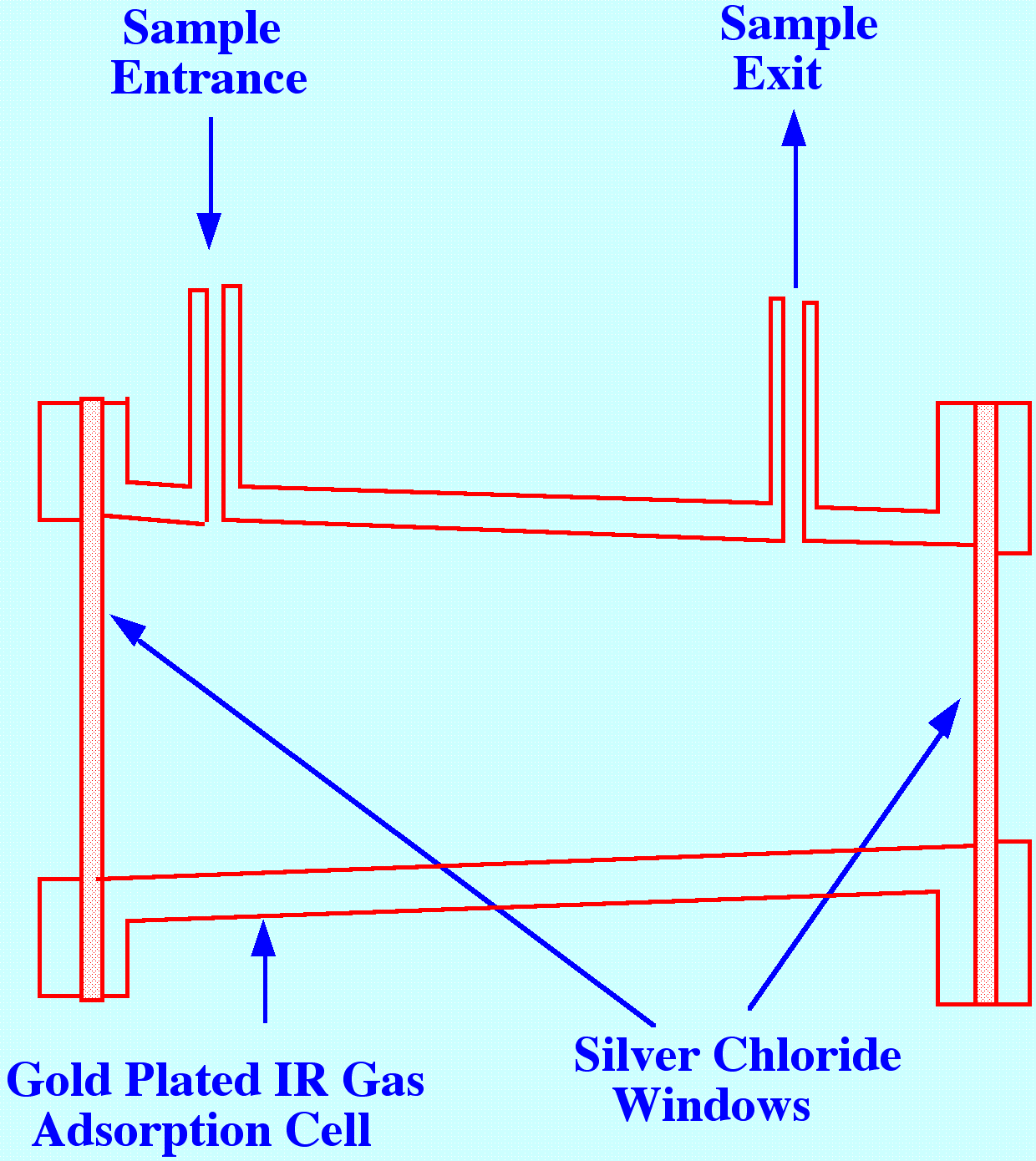
Gas
samples are often of interest in pollution studies and spectra can be
obtained from such samples by employing a gas sample cell similar to
that shown in figure 14. The cell is gold plated and polished
internally to reduce light loss and increase absorption of the
infrared light. The windows were constructed from silver chloride
(horn silver ) to provide mechanical strength and rigidity, and also
to be relatively impervious to water vapour. This type of cell was
used in the early gas chromatography and in IR spectroscopy tandem
systems. Another type of gas cell that can provide very high
sensitivity and, thus, spectra from very small amounts of sample is
the light pipe. This device was originally designed to provide on
line spectra from peaks eluted from a gas chromatography capillary
column where the amount of solute eluted may be less than one
microgram.
Light
pipes were introduced by Wilks and Brown in 1964 [3] and consist of
tubes of circular or rectangular cross-section with highly reflecting
internal surfaces that are usually produced by gold plating. A
diagram of the optical system of the Perkin Elmer instrument, which
may be considered typical for a light pipe gas sampler, is shown in
figure 15.
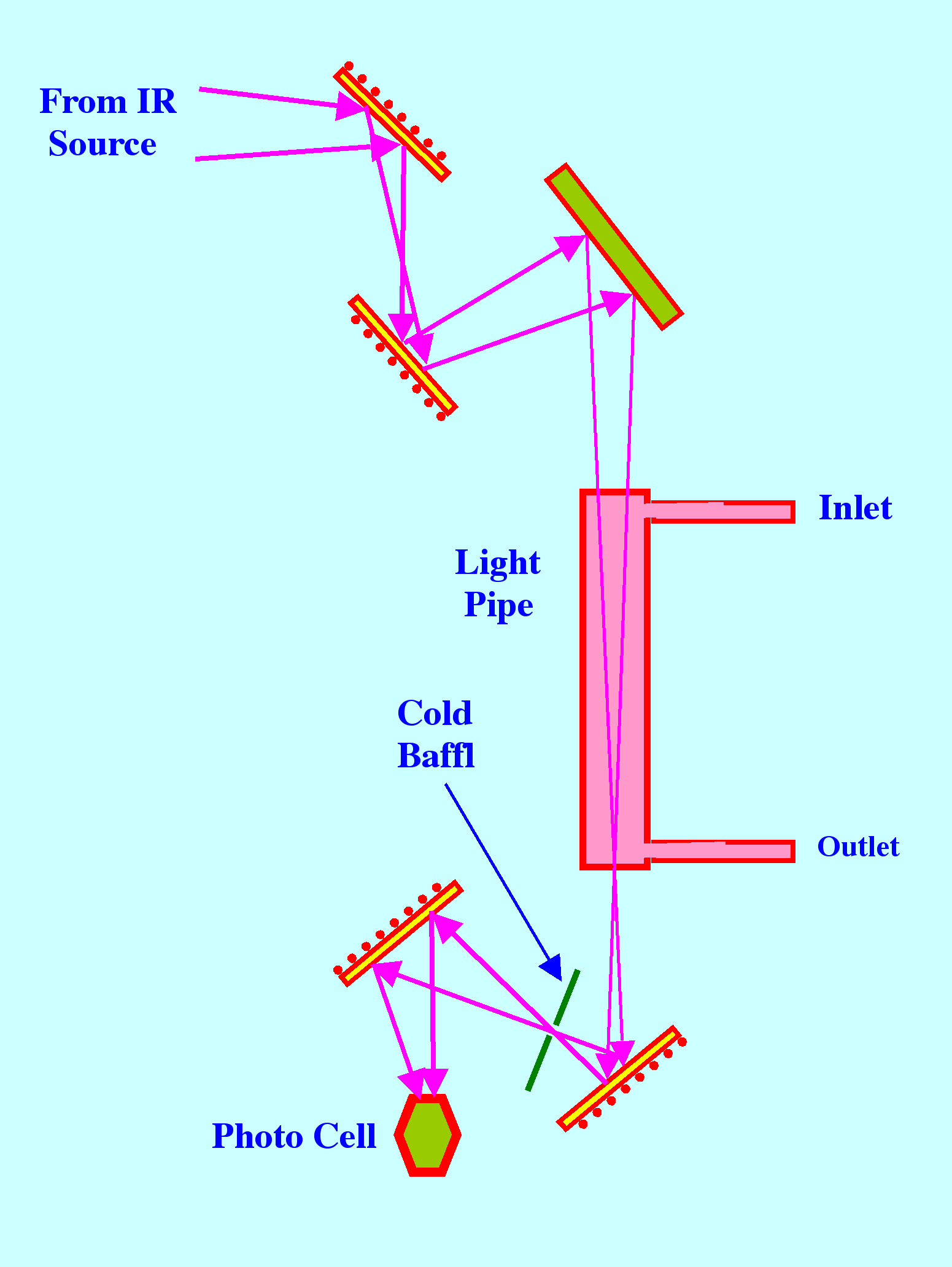
The
light source is moved back, so that the focus, which normally
coincides with the entrance of the light pipe, is transferred to the
exit of the light pipe, but the process is not completely efficient.
Internal reflections at the walls of the light pipe results in the
'apparent path length' being increased by about 33%. Many modern
GC/IR systems employ Light Pipes to augment the IR signal.
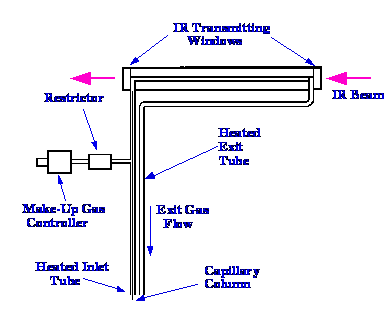
A
diagram of a light pipe originally designed by the Perkin Elmer
Corporation is shown in figure 16. The light pipe itself is 120 mm
long, 1 mm I.D and coated internally with gold. The oven surrounding
the light pipe, can be operated up to
350⁄C, and is carefully designed to eliminate any cold spots on
the tube, which might allow solute condensation (the elimination of
any possibility of sample condensation is extremely important). In
normal use for gas sampling, the sample is passed into the
inlet tube and out of the exit tube
in the normal manner. When used as GC/IR interface the capillary
column is led into the interface through a heated tube right up to
the actual light pipe. Concentric to the column, and through the same
heated tube is fed a stream of scavenging gas that carries the solute
through the IR light pipe. This maintains the integrity of the
separation at the expense of some solute dilution and consequent
slight loss of sensitivity. If the solute bands were not swept out by
the scavenging gas, the solute peaks from the column would accumulate
in the IR light pipe, and as a consequence, several solutes would be
detected and measured simultaneously, and resolution would be lost.
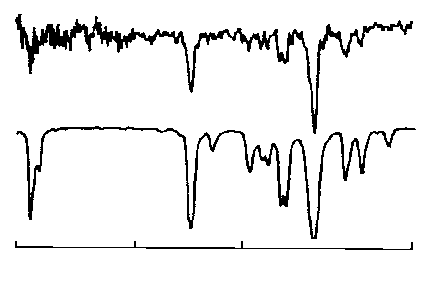
It
is claimed that satisfactory spectra can be obtained from a 1-5 ng of
material in the light pipe. This claim is supported by the Spectrum
shown in Figure 17.
The
Spectrum from 10 ng of material has sufficient IR absorption data to
allow the identity of the solute to be confirmed by comparison with
the library Spectrum shown below the sample Spectrum. Clearly, the
system can be used to, either aid in structure elucidation, or
confirm compound identity.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.









