ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
The Use of the Zeeman Effect to Reduce Background Interference
Background
interference is caused by either, non-specific absorption arising
from light scattering caused by solid particles or liquid droplets in
the atomizing cell or, by light absorption caused by molecules or
radicals originating in the sample matrix. To compensate for
background absorption the background absorption is usually measured
by separate experiment and subtracted from the absorption of the
sample solution. One method for eliminating background absorption is
by exploiting the Zeeman
Effect.
The
Zeeman
effect
is the splitting of spectral lines into several polarized
components as a result of the effect of an applied magnetic
field.
In fact, it is analogous to the Stark
effect
where a spectral line is split into a number of separate lines as a
result of the application of an electrostatic
field.
On the application of the magnetic field a central line appears at
the same wavelength as the original line (the π
line) having half the intensity of the original line. On either side
of the π
line appears two other lines (the σ±
lines) having one quarter of the intensity of the original line. The
π
line is linearly
polarized
with the electric vector parallel
to
the magnetic field
and the σ±
lines are circularly polarized (rotating in opposite directions)
about the lines of force with their electric vector linearly
polarised at
right angles
to the direction of the magnetic field. In certain elements (e.g.
sodium and silver ) the π
and σ±
are
further split
into a number of lines; the
sodium
D1
line
splits into four lines (2 π
lines and 2 σ±
lines) and the D2
line of the sodium into 6 lines (2 π
lines and 4 σ±
lines). The use of the Zeeman Effect in Atomic Spectroscopy is based
on the fact that background absorption due largely to molecular
scattering is not affected by the presence of a magnetic field.
A magnetic field can be
applied to either the radiation source (source-shift
Zeeman background correction)
or to the atomizing cell (analyte-shift
Zeeman background correction).
The
field can be applied transverse or longitudinal to the optical path.
The polarizer can be placed before or after the atomizing cell. In
source-shift Zeeman background correction
the spectra; source line is split into π
lines and σ±
lines and on passing through the atomized sample the π
line is absorbed by both sample and back ground whereas the σ±
components are only absorbed by the background. The technique can be
employed with either flame or electrothermal ionization techniques.
In analyte-shift Zeeman
background correction the atomizing cell is placed in the magnetic
field. A diagram of an analyte-shift Zeeman background correction
instrument is shown in figure 24.
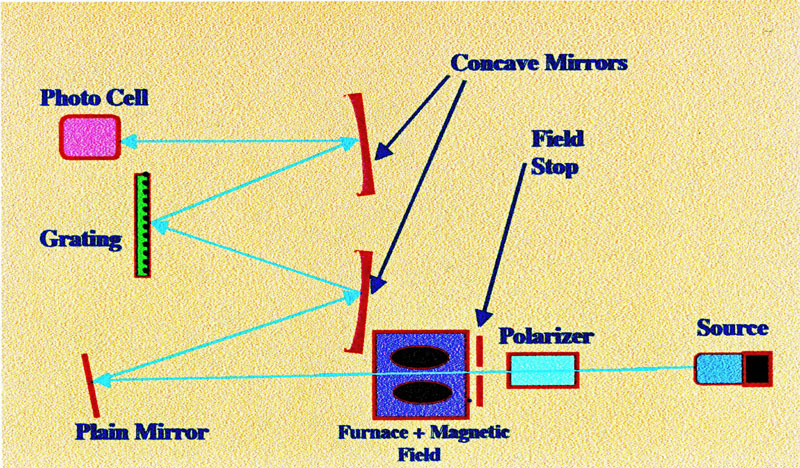
Light from
the source passes through a polarizer that splits the radiation into
two linearly polarized beams, parallel and perpendicular to the
direction of the magnetic field. These beams pass alternately through
the atomic vapour in the atomizing cell. During the first cycle the
polarized light parallel to the magnetic field is absorbed by the
sample and attenuated by the background. In the second cycle the
polarized light perpendicular to the magnetic field passes through
the sample cell and only the background attenuation is measured.
Thus, from the two sets of data the true exclusive absorption by the
sample can be determined.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



