The Electrospray Liquid Sampling System
The electrospray ion-producing
inlet
system is probably the most commonly used interface handling liquid
sample solutions. An early report describing this
interface
was that by Whitehouse et al.
[17], The
explanation given for ion formation is as follows. Electrospray
ionization occurs as the result of strong electric field acting on
the surface of a sample solution as it is sprayed into a dry gas such
as nitrogen . This process produces a cloud of charged droplets that
rapidly evaporate and, as a consequence. shrink in size. The
accompanying increase in charge density that results from the
decrease in volume, surface area, and radius of curvature of the
droplets causes very
strong
electric
fields
to be formed. As each drop continues to shrink, the electric fields
become sufficiently strong to cause the droplets to explode,
producing ions. Due to the strength of the electric field, and the
large number of ions that are produced, many of the ions that are
formed will contain multiple charges. It will be seen later that as
the mass spectrometer discriminates on the basis of the m/z value of
the ion, multiple ion production will, in effect, extend the mass
range of the spectrometer.
A diagram of the Hewlett-Packard
Electrospray Ionization LC/MS interface is shown in figure 23.
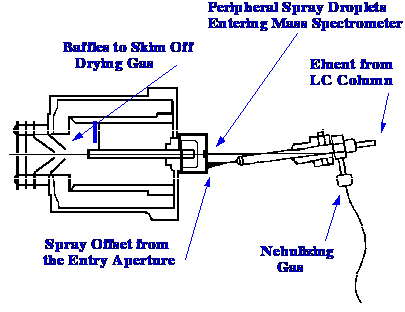
Courtesy of the Hewlett-Packard
Company.
The sample solution is mixed
with a
nebulizing gas and the spray jet directed onto a disk target. The
target is set at a high potential relative to that of the spray
nozzle. In the center of the target is a pinhole entry into the
source and the jet is directed slightly to the side of this aperture.
The fine droplets at the edge of the spray are drawn into a chamber
(held at a reduced pressure) through the pinhole aperture. Inside the
chamber, the droplets are entrained in a stream of hot nitrogen gas.
The hot gas rapidly evaporates the solvent, producing ions in the
process described above. The core of the jet is skimmed by a set of
conical screens to selectively remove the drying gas so that the ions
pass directly into an ion-optical arrangement. The ion optical system
directs the ions, so formed, into the mass analyzer. In this system,
ions with multiple charges are produced as a result of a molecule
becoming associated with more than one proton. As a result, an ion of
molecular weight 1000, carrying three charges, will appear at an m/z
value of 333.3 on the Spectrum where an ion of mass 333.3 and unit
charge would normally appear. It is clear that the production of
multiple charged ions, in effect, increases
the mass range of the spectrometer. As an
example of the
use of the ion spray used as an LC/MS interface, the total ion
current chromatogram of a sample from the tryptic digest of lysozyme
is shown in figure 24.
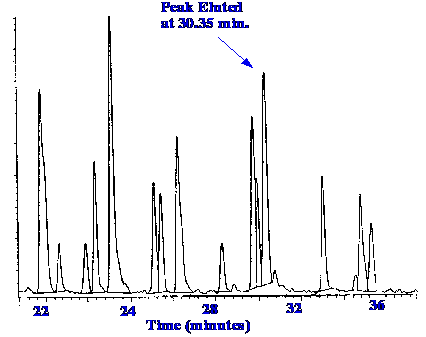
Courtesy
of the Hewlett
Packard Company.
The
mass spectrometer employed was the Hewlett-Packard MS Engine
Quadrupole Mass Spectrometer. The mass Spectrum of the peak eluted at
30.35 minutes is shown in figure 25.
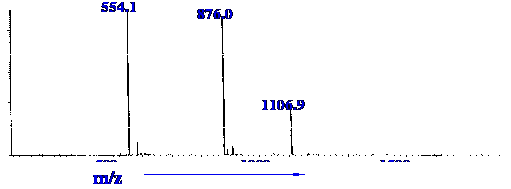
Courtesy of the Hewlett-Packard
Company.
Figure 25 displays three peaks
at m/z
values of 554.1, 876.0 and 1106.8. It is seen from figure 25 that the
peak was complex and all the components were not resolved from one
and other. The question arises did all three peaks originated from
the same solute, or did any pair of the peaks result from multiple
charges on the same molecule. A program is included in the
Hewlett-Packard data handling software that tests the
inter-relationship between the individual peak masses in the mass
Spectrum. In practice the software determines whether the molecular
weights of any pair or group of peaks are linearly related to each
other. If the respective peaks increase or decrease proportionally
with one another, then they must originate from the same parent
molecule, and probably represent multiple charges on the same
molecule. The peaks in figure 25 were tested in this manner, and the
results from the correlation program are shown in figure 26.
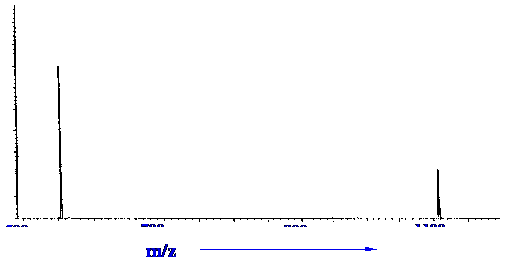
Courtesy of the Hewlett-Packard
Company.
The
data demonstrated that the peak at 876.0 m/z was not related to the
other two and, furthermore, the peaks at 554.1 and 1106.9 were doubly
and singly charged species of the same ion.
e.g. (1106.0-1(H+))/2
+
2((H+) = 554.95 cf 554.1 (from Spectrum)
Unfortunately, the system, in
some forms,
does have some certain disadvantages. The electrospray system may not
work well with solvents having high water content, but certain
interface designs work better than others with aqueous mixtures. Some
electrospray nebulizers are designed specifically to cope with
solvents of high water content. It follows that the electrospray
system must be chosen to suit the particular analyses for which it is
to be used. Its area of application has increased rapidly over the
past years, and some particular applications that illustrate its
versatile capabilities will be described.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.






