Reflectance Methods
Reflectance
techniques are used for those samples where IR transmission is
difficult or impossible. There are two types of reflectance
measurements, attenuated total reflectance where and internal
reflectance cell is employed and External Reflectance where the IR
beam is reflected directly from the sample surface.
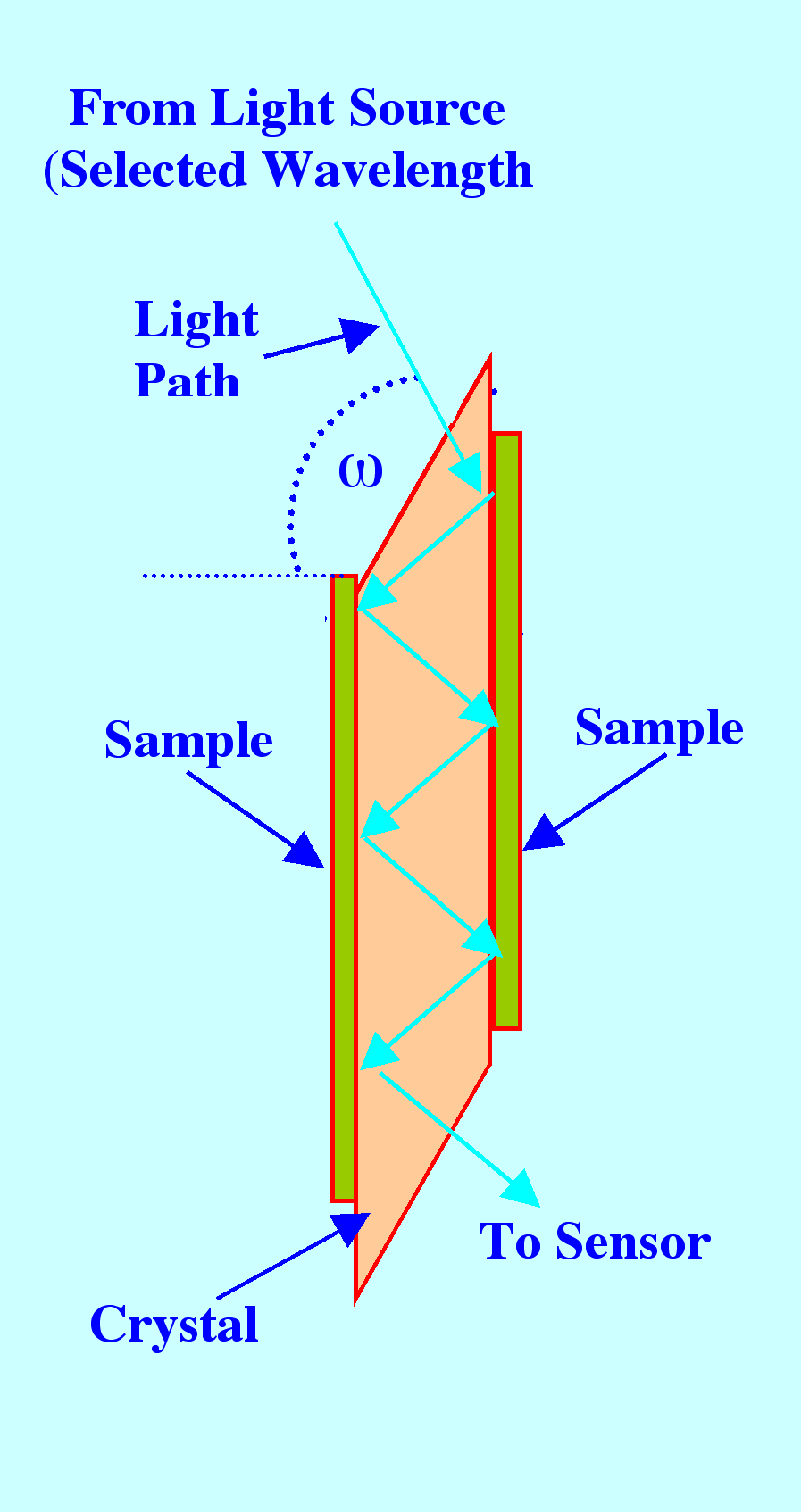
Attenuated
Total Reflectance Spectroscopy
Attenuated
total reflectance employs total internal reflection as shown in
figure 18. A light beam entering a crystal will undergo complete
internal reflection if the angle of incidence is greater than the
critical angle, which will be a function of the refractive indices of
the two surfaces.
On
striking the surface the beam will penetrate the surface slightly
and, if the substance absorbs light at the wavelength of the incident
light, some of the light will be absorbed. The intensity of the
attenuated radiation can then be plotted against the wavelength of
the incident light and an absorption Spectrum will be obtained.
The
depth of penetration (dp)
will be a function of the wavelength (λ),
the refractive index of the crystal, and the angle of incident
radiation (ω)
and is given by the following equation,
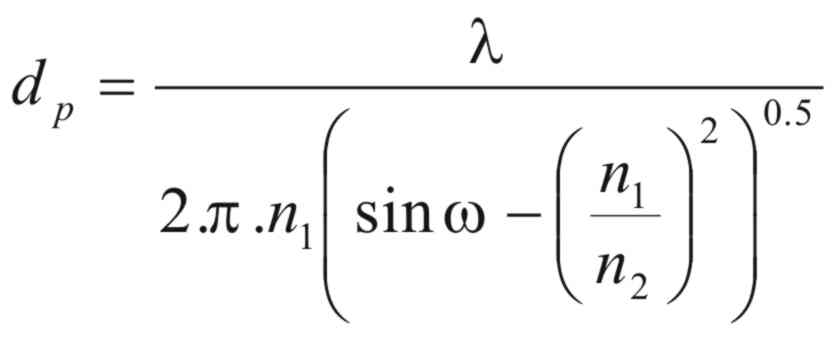 (6)
(6)
where
(n1)
and (n2)
are the refractive Indices of the sample and the crystal
respectively.
Typical
materials that can be used as crystals for total reflectance
spectroscopy are zinc selenide (RI 2.4, 20000-500 cm-1), germanium
(RI 4.0, 5000-550 cm-1) and thallium /iodide (RI 2.4, 17000-250 cm-1).
The
common factors between all the materials are that they are relatively
insoluble in water and have high refractive indices.
Multiple
Internal Reflectance
Multiple
internal reflectance techniques produce more intense spectra as a
result of multiple reflections.

In
contrast to attenuated total reflectance that usually employs a
prism, Multiple Internal Reflectance techniques employ specially
shaped crystals that allow multiple reflections. Such a crystal is
shown in figure 19. Such crystals may produce as many as 25 multiple
reflections.
External
Reflectance Techniques
If
radiation is focused on the external surface of a sample two forms of
reflectance can occur. The first is specular
reflectance and
the second diffuse
reflectance.
Both forms of reflectance can be used to produce spectra from a
sample. In order to have effective and useful reflectance, the
surface must be reflective or be attached to a reflective backing.
This
technique has been applied to the examination of surface coatings
such as paints polymers and metal surface coatings.
Specular
Reflectance
If
the reflected light from the surface is measured where the angle of
reflection equals the angle of incidence then Specular Reflectance is
said to occur. The amount of reflected light in Specular Reflectance
is a function of the angle of incidence, the refractive index of the
sample, the surface roughness and the adsorption properties of the
sample. If grazing angles of incidence are employed, this, in effect,
increases the path length through the coating or surface and
increases the sensitivity. Grazing angles of up to 85o
can be employed. If the coating on the reflective surface is one
micron or more thick, then the incident and reflective angles are
usually about 30o.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.


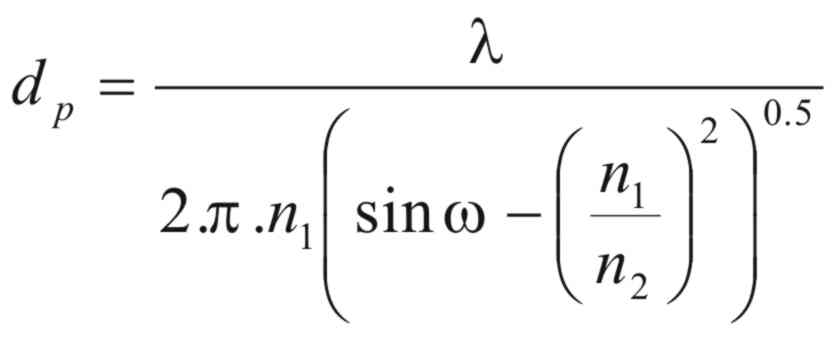 (6)
(6)

