ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Synchrotron Sources for Raman Spectrometry
The
synchrotron is a relatively novel source of IR radiation. The basic
principle of the synchotron is depicted in figure 8.
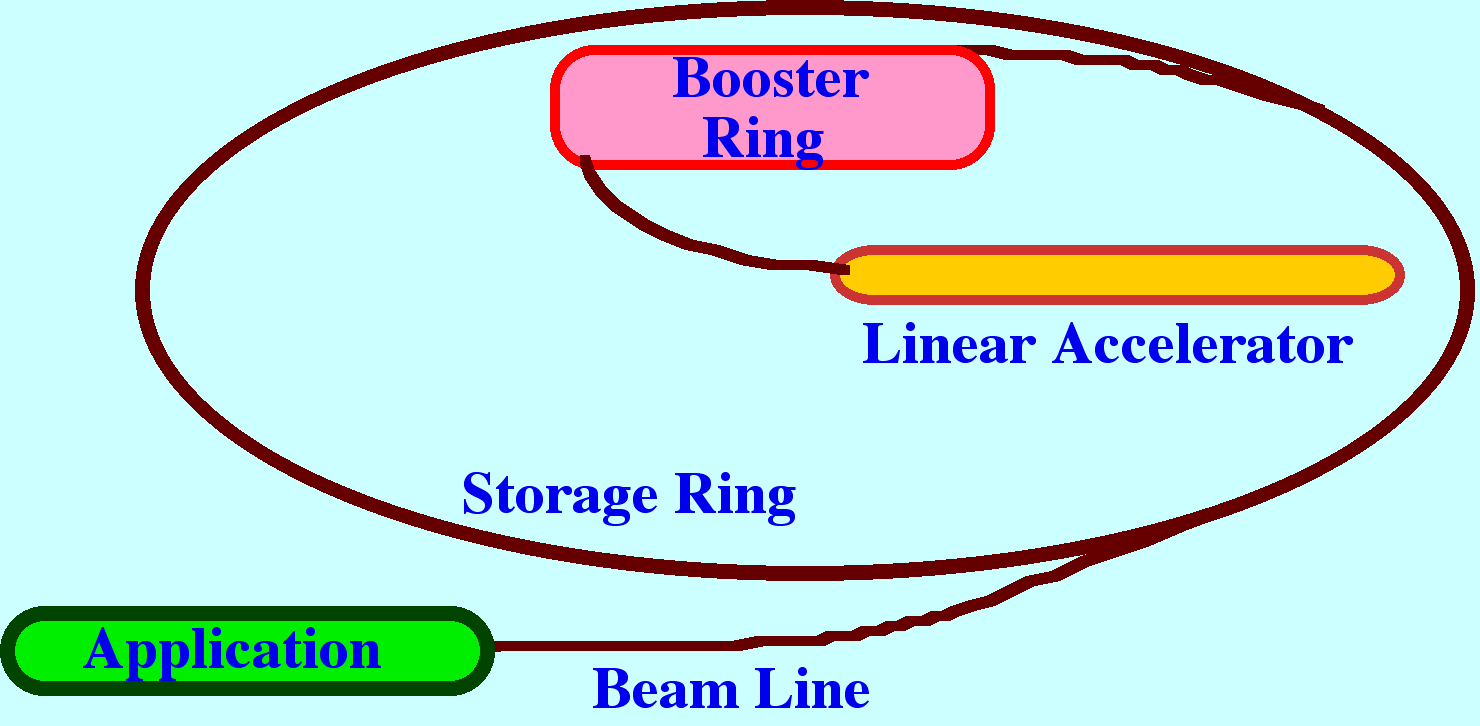
A
beam of electrons is generated in a linear accelerator that then
enters a ‘booster ring’ where the electrons are
accelerated to close to the speed of light. The electrons are then
injected into a larger ring (the storage ring) where the electrons
continue to circulate at relativistic velocities. Any charged
particle, travelling at these velocities and traversing a curved path
will emit intense broad-band electromagnetic radiation across the
whole Spectrum from X-rays to the far infrared. Modern synchrotons
utilize magnetic devices called wrigglers”, and “undulators”
round the ring to increase the intensity of the radiation. The
radiation is emitted tangentially to the electron path and so any
port incorporated in the ring will provide an intense radiation beam.
The beam is then sent through an optical system where the wavelengths
of interest are separated and made available for experiments. Most
experiments carried out with radiation from a sychrotron involve
X-rays but some work on infrared has been carried out. Time can be
rented from the synchrotron establishment but it may be many years
before such facilities become generally available. Such a source does
hold promise for vastly improved results from Raman Spectroscopy. In
figure 9 the performance of a typical FT-Raman instrument is shown by
the spectra of indene.
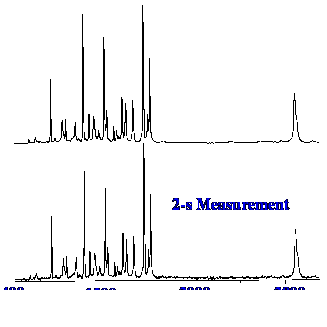
The
excitation energy was 1 W and data was acquired for two seconds in
the lower Spectrum and for thirty seconds in the upper Spectrum. The
two spectra appear identical which demonstrates that no thermal
degradation occurred with the extended exposure to radiation for
thirty seconds. The majority of samples can cope with high intensity
near-IR without decomposition as the absorption is involved with
combinations of vibrational modes and overtones and, therefore, are
rather weak.
The
main advantage of working with near-infrared radiation is the lack of
Fluorescence that can seriously interfere with the quality of the
resulting Spectrum. This is clearly demonstrated by the two spectra
of anthracene obtained employing radiation in the Visible region
(5145Å) and in the near-infra red (1.06μm)
shown in figure 10. The upper Spectrum produced by irradiation with
Visible light shows the spectra superimposed on a broad ‘hump’
of Fluorescence almost completely obscuring the Raman Spectrum,
whereas the lower Spectrum obtained by near-infrared irradiation
gives a clean, virtually Fluorescence-free Spectrum.
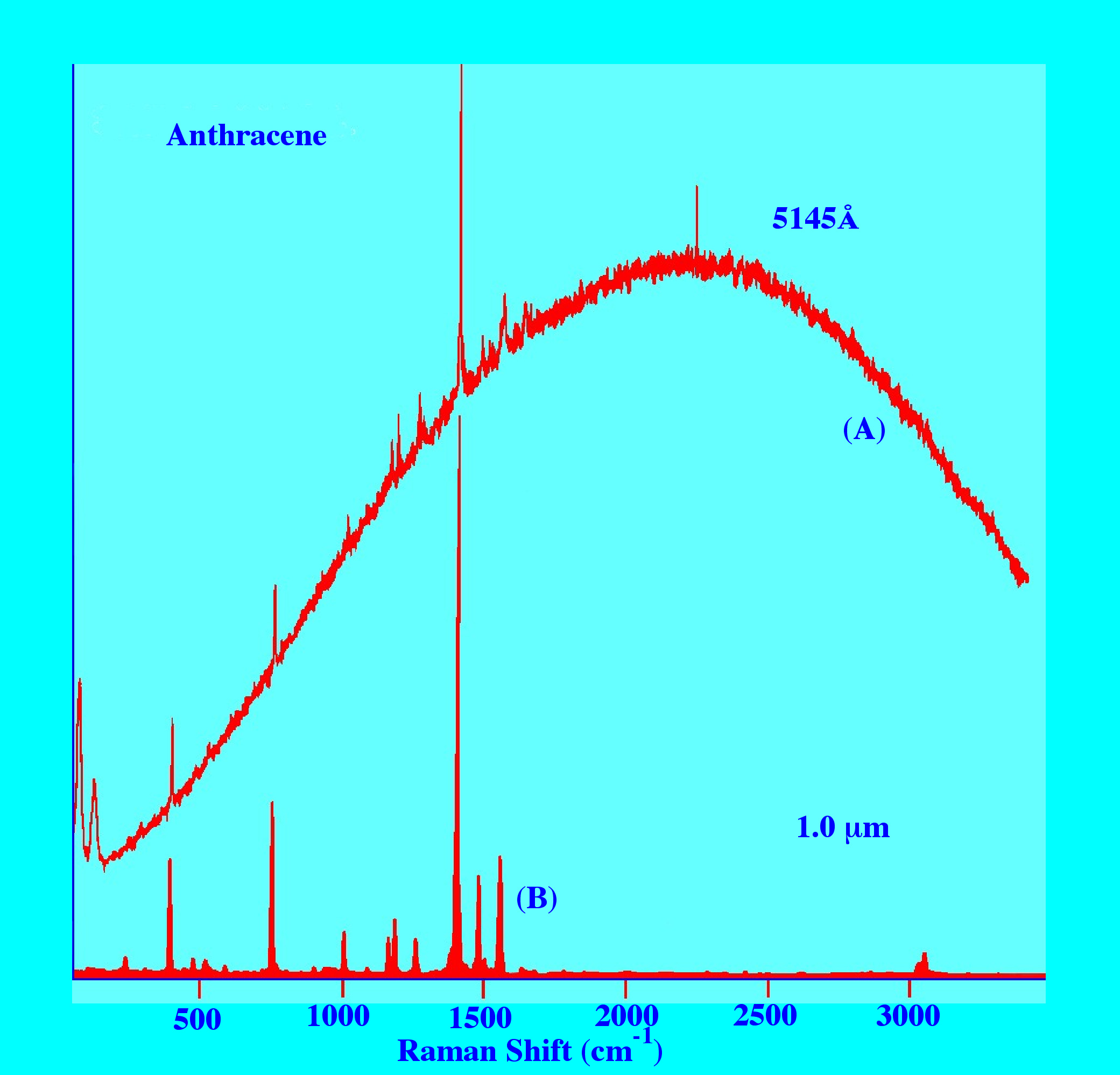
Admittedly,
this may well be one of the worst-case ‘scenarios’;
nevertheless the spectra clearly demonstrate the advantages of
employing near-infrared radiation in the production of Raman spectra.
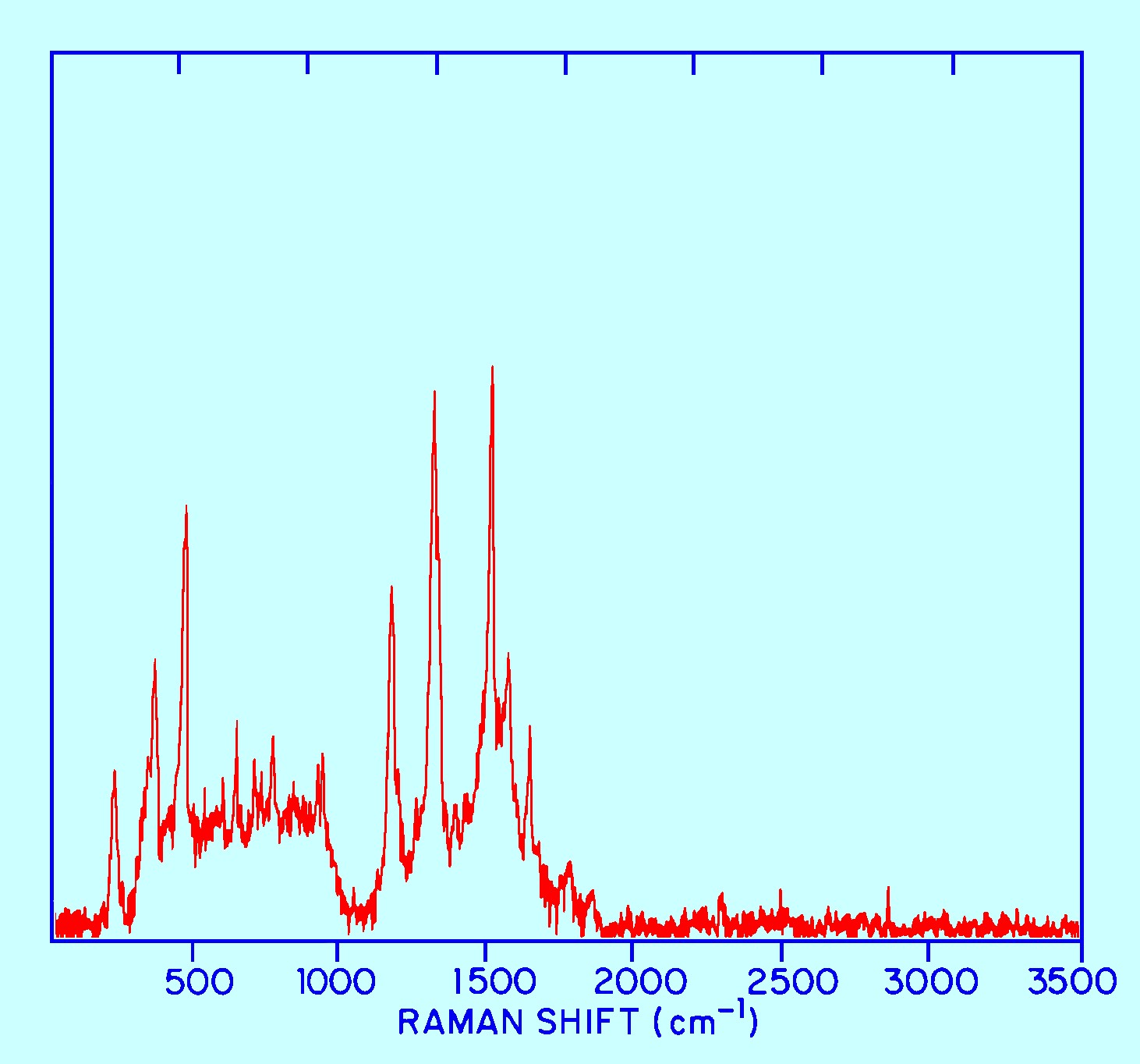
A possibly even more impressive advantage of the use of the near
infrared for the excitation to eliminate Fluorescence is shown in
figure 11.
In
this case the Raman spectra is being taken of a strongly fluorescent
material but employing radiation of 106 mm as the radiation with a 60
second measurement time a Raman Spectrum of a useful form could still
be obtained.
Employing near-infrared
frequencies as the exciting radiation in conjunction with FTIR
techniques provides yet another advantage. The ease and accuracy of
spectra addition and subtraction employing the FTRI system used in
conjunction with spectra that have extremely low Fluorescence
interference allows solvent spectra subtraction techniques to be
extremely accurate.
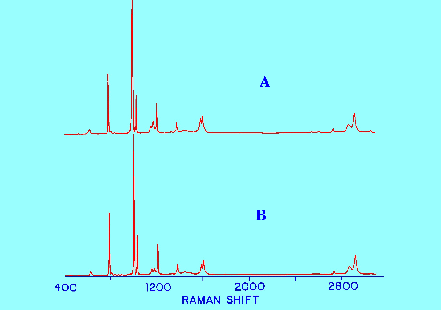
Spectrum
A obtained from a 20% solution of toluene in benzene ,
Spectrum
B obtained from pure toluene.
As
both spectra are (in a sense) extremely ‘pure’,
subtracting the solvent Spectrum from the mixed Spectrum provides a
very accurate
Spectrum
of the solute
alone that
can be used with confidence
for both identification
purposes
and also for structure elucidation.
The
spectra given in figure 12 clearly demonstrate this advantage. The
Spectrum obtained after subtracting the Spectrum of the solvent from
the Spectrum of the solution of the solute in the solvent is
virtually identical to the Spectrum obtained from a sample of pure
toluene. In fact the difference is almost indistinguishable.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.







