The
Quadrupole Mass Spectrometer, either as a single quadrupole or as a
triple quadrupole can also provide MS/MS spectra (at technique that
will be discussed later. A diagram of a Quadrupole Mass Spectrometer
is shown in figure 68.

The operation of the quadrupole
mass
spectrometer is completely different from that of the sector
instrument. The quadrupole spectrometer consists of four rods that
must be precisely straight and parallel and so arranged that the beam
of ions is directed axially between them. Ideally the rods should be
hyperbolic in cross section but in practice less expensive
cylindrical rods are nearly as satisfactory. A voltage comprising a
DC component (U) and a
radio frequency
component (Vocosωt)
is applied between adjacent rods, opposite rods being electrically
connected, as shown in figure 68. Ions are accelerated into the
center, between the rods, by a relatively small potential ranging
from 10 to 20 volts. Once inside the quadrupole, the ions oscillate
in the (x) and (y) dimensions as a result of the high-frequency
electric field.
The stability of the oscillating
ions are
determined by the magnitude of two parameters (a)
and (q) which are
defined by the
following equations:
a
=
8eU/(mr02w2) and q = 4eVo/(mr02w2)
where, (r0)
is half the distance between opposite rods of the quadrupole system
and the other symbols have the meaning previously ascribed to them.
Theory
predicts that oscillations of the ions will only remain stable for
certain combined values of (a)
and (q).
Outside these values the oscillations become infinite and the ions
will strike the rods and become dissipated. The relationship between
(a) and (q)
is shown in figure 69
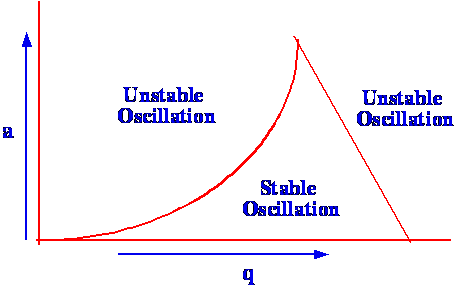
It is seen that there is a very
restricted
range of values of (a)
and (q)
that can permit the mass spectrometer to operate in a stable mode.
The mass range is scanned by changing both (U)
and (V0)
while keeping the ratio (U/V0)
constant.Quadrupole Mass Spectrometer
is
compact, rugged and easy to operate and consequently is a popular
instrument for general use in mass spectrometry.
Unfortunately, the mass range of
the
quadrupole spectrometer does not extend to such high values as the
sector instrument but as already has been discussed, under certain
circumstances multiple charged ions can be generated and identified
by the mass spectrometer. This, in effect, significantly increases
the effective mass range of the device.
The Quadrupole Mass Spectrometer
can also
be constructed to provide MS/MS performance. This is achieved by
combining three quadrupole units in series. A
diagram of a
triple Quadrupole Mass Spectrometer is shown in figure 70.
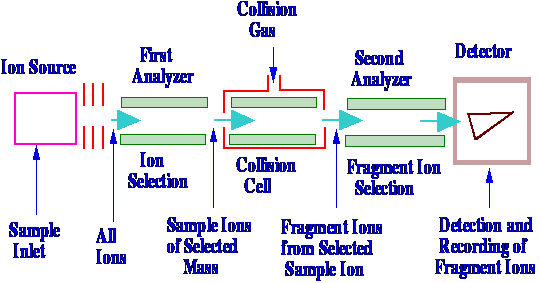
The sample enters the ion source
and is
usually fragmented by either an electron impact or chemical
ionization process. In the first analyzer the various charged
fragments are separated in the usual way and then pass into the
second quadrupole section sometimes called the collision cell.
The first quadrupole behaves as a straightforward mass spectrometer.
Instead of the ions then passing
to a
sensor, they pass into a second mass spectrometer. In this way a
specific ion can be selected by the first quadrupole for further
study. In the center quadrupole section, the selected ion is further
fragmented by collision ionization
and the new
fragments then pass into the third quadrupole which functions as a
second analyzer. The second analyzer segregates the new fragments
into their individual masses, which are detected by the sensor,
producing the mass Spectrum (originally from ions of one mass only).
In this way, the exclusive mass Spectrum of a particular molecular or
fragment ion can be obtained from the myriad of ions that may be
produced from the sample in the first analyzer. It is seen that this
can be an extremely powerful analytical system that can handle
exceedingly complex mixtures and very involved molecular structures.
The system has more than adequate resolving power and is valuable for
structure elucidation.
Most modern quadrupole mass
spectrometers
are employed in conjunction with a separation unit such as a
chromatograph. Due to the compact nature of the mass discrimination
unit (compared with a Sector Mass Spectrometer) the combination of a
chromatograph and a Quadrupole Mass Spectrometer can be manufactured
as a bench top instrument.
A photograph of the triple
quadrupole mass
spectrometer tandem system consisting of a gas chromatograph combined
with a mass spectrometer which was designed for drug analysis and
manufactured by Varian Inc. is shown in figure 71.
The instrument is fitted with
dual
injectors and can provide both electron impact and chemical
ionization facilities and MS/MS features if required.

Courtesy
of Varian Inc.
Another example of the many
tandem
instruments that are available is that shown in figure 72. This also
is designed round the Saturn 2200 quadruple mass spectrometer
including a gas chromatograph.
Courtesy
of Varian Inc.
This instrument is fitted with
an auto
sampler in addition to the gas chromatograph, dual injectors and a
second GC detector such as the electron capture detector.
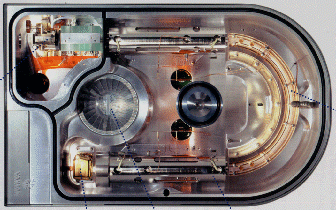
A photograph of the layout of
the triple
mass discrimination unit is shown in figure 73, In the top left hand
corner is the combined electron impact and Chemical Ionization
sources with a hexapole ion guide interface to the mass analyzer unit
that significantly improves the performance of the ionization process
(improves the ionization efficiency by ensuring more ions that are
formed enter the mass analyzer). Slightly left of center is a single
turbo pump providing differential pumping for the source and the
analyzer compartment. After the ions are produced they are conducted
by the hexapole ion guide into the first quadrupole where the initial
mass discrimination takes place. After selecting the ion(s) of
interest the selected ions pass into the second quadrupole or
collision cell. The collision cell is curved which ensures efficient
dissociation. The selected ion(s) then pass into the third mass
analyzer. Pre- and post-ion guides improve the transmission
efficiency of each quardrupole. No lenses are employed which
simplifies the tuning and further improve the magnitude of the
signal. The ions after discrimination are accelerated by a 5kV
gradient, which provides the same ion conversion efficiency as a ±
15kV dynode without suffering the noise associated with
dynodes.
Courtesy
of Varian Inc.
The
operating conditions of the mass analyzers and their associated
equipment are continually available to the analyst in the form of a
chart as shown in figure 74.
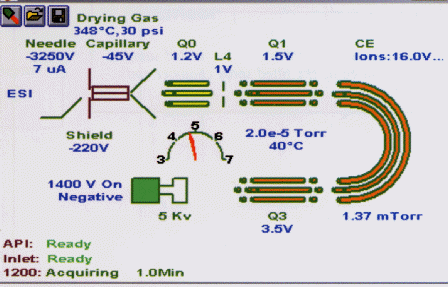
Courtesy
of Varian Inc.
The
action of the triple quadrupole system is depicted by the mass
spectra shown in figure 74A, 74B, and 74C provided by Varian Inc. The
many ions from the ionization source (and these will be parent ions
of the substance(s)) are fed into the sample system together with any
fragment ions and these will be separated in the first mass analyzer
(i.e. the first quadrupole). The number of charged
fragments
produced will depend on the ion source. If the ions are produced by
electron impact then many charged fragments are likely to be
produced. If the ions are formed by Chemical Ionization then any ions
formed are all likely to be parent ions or simple ‘charged
addition’ ions. Thus, the mass discrimination afforded by the
first quadrupole analyzer is shown in figure 74A.
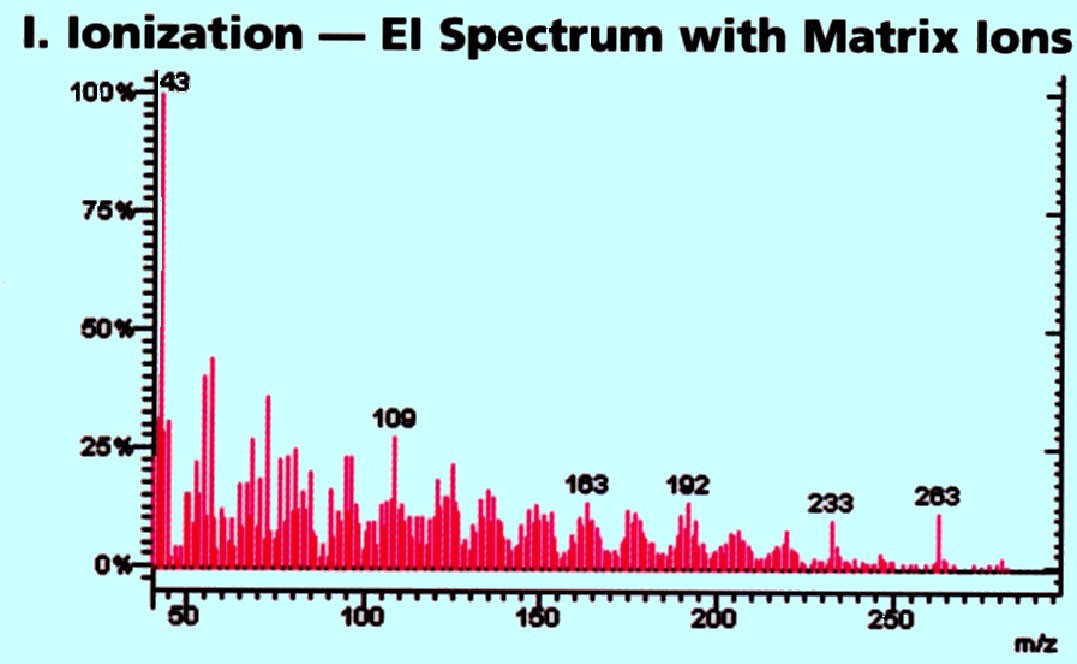
Courtesy
of Varian Inc.
This
allows a specific ion (or group of ions having the same m/z values)
to be selected and passed into the second analyzer. In figure 74 A
the ions of m/z 263 pass into the second analyzer. This is depicted
in the spectra shown in figure 74 B
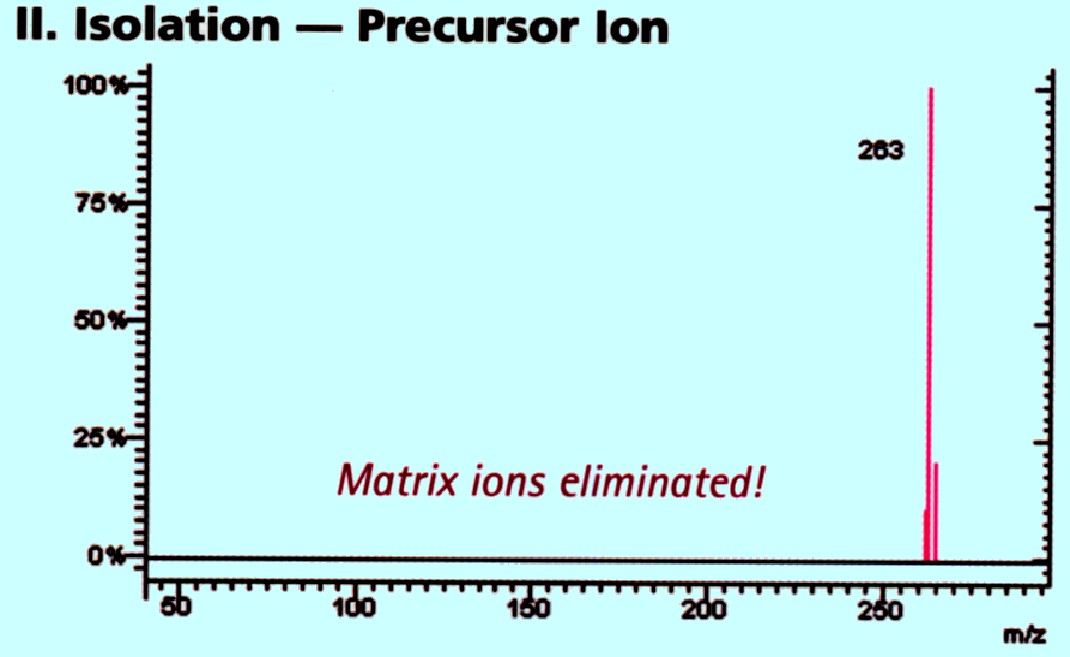
Courtesy of Varian Inc.
In the second quadrupole collision
induced
fragmentation takes place and the products pass into the third
quadrupole where the fragments are mass analyzed providing the
Spectrum shown in figure 74C
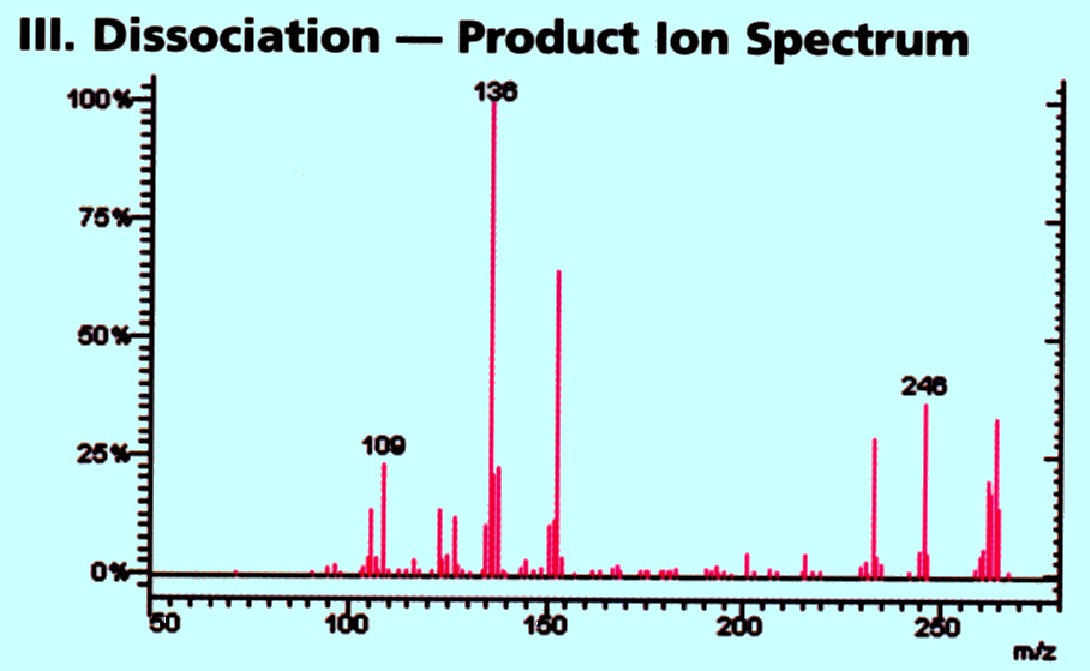
Courtesy
of Varian Inc.
The unique collision induced fragments
are
presented against a virtually noise free background and yield better
quantitative date and more informative data for structure
elucidation. The triple quadrupole system, as well as providing more
confident compound identification also provides high sensitivity. An
example of the application of the system to drug analysis is shown in
figure 75
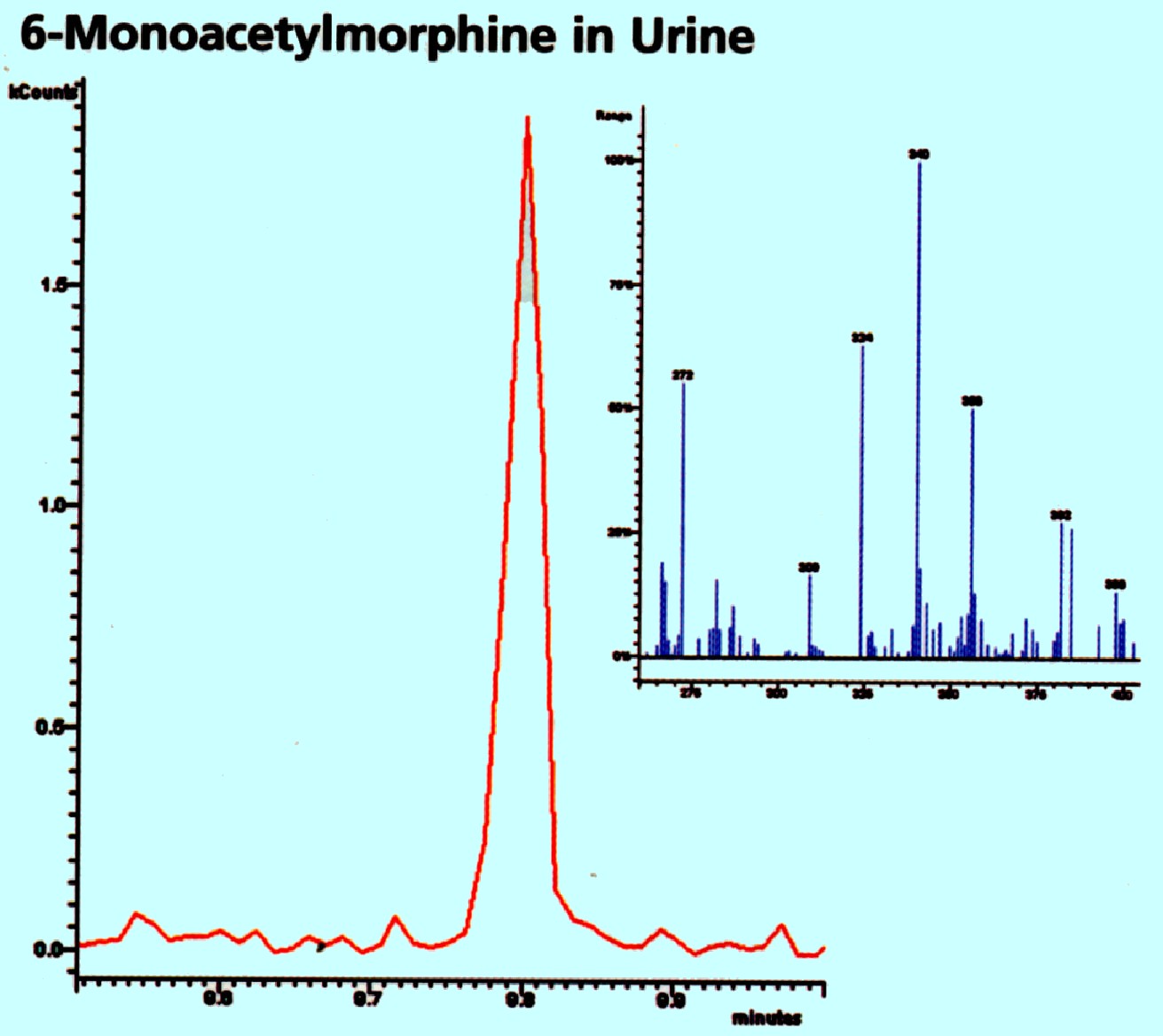
Courtesy
of Varian Inc.
The
Combination of the triple quadrupole
mass spectrometer with a separation technique such as a gas
chromatograph or a liquid chromatograph is probably one of the most
powerful analytical tools available to the contemporary chemist.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.













