Normal Vibration Modes
A
molecule can be regarded as an assembly of balls (atomic nuclei) and
springs (chemical bonds). The atoms of any molecule with have three
coordinates that define its position and potential directions of
movement in space and therefore if the molecule has (n)
atoms then it will have (3n)
translational
degrees of freedom.
However, if the position of an atom is defined then the bond distance
and angle will also be fixed.
Now
the molecule as a whole can move in the three coordinate directions
and this will utilize three of the degrees of freedom, thus, there
will remain (3n-3)
degrees of freedom. In the same manner the rotational movement of a
non-linear molecule can be resolved into three components and
specification of these axis also utilizes three degrees of freedom so
the so the total degrees of freedom become (3n-6)
Thus
a nonlinear
molecule can have
(3n
– 6)
fundamental
vibrations.
However,
a linear molecule has an exceedingly low energy when rotating about
its bond axis and can be considered to only have two rotational
degrees of freedom,
Thus,
a linear
molecule can have (3n
– 5)
fundamental vibrations
In
the case of a diatomic molecule (number of atoms is 2, (i.e.n=2),
it can only have one
degree of vibrational freedom as the joining bond can only stretch or
compress. It will also have three
degrees of translational freedom and two
degrees of rotational freedom Thus, the diatomic molecule will have
six degrees of freedom (i.e.3n =
3x2 = 6, as n=2).
As
already considered, there can be two basic forms of a molecule, a
non-linear
molecule and a linear
molecule; a linear and non-linear molecules are typified by carbon
dioxide and water and these are shown in figure 3.

Carbon
dioxide and water each have three degrees of translational freedom.
Water has three degrees of rotational freedom but the linear carbon
dioxide molecule has only two degrees of rotational freedom as the
molecule rotating about its O-C-O axis involves little or no energy.
Thus, the degrees of freedom for vibrational energy will be 3n-5 for
carbon dioxide and 3n-6 for water. This relationship is summed up in
table 1.
Table
1. The Distribution of Degrees of Freedom for Polyatomic Molecules
| Degrees of Freedom |
Linear Molecules |
Non-Linear Mol;ecules |
| Translational |
3 |
3 |
| Rotational |
2 |
3 |
| Vibrational |
3n-5 |
3n-6 |
| Total |
3n |
3n |
The
frequency of a vibrational mode will depend on the mass at either end
of the bond and the stiffness of the bond. The stiffness of the bond
is defined by a proportionality constant called the force constant
(k).
The
frequency The frequency of the adsorbing wave (ν)
is given by,
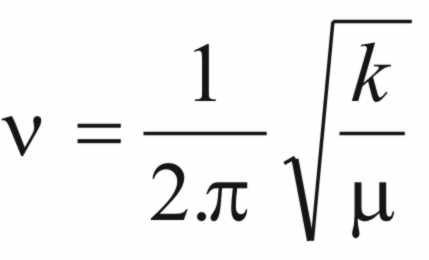 ( 2 )
( 2 )
The
constant (μ)
is the reduced mass of the system and is calculated from the
following equation
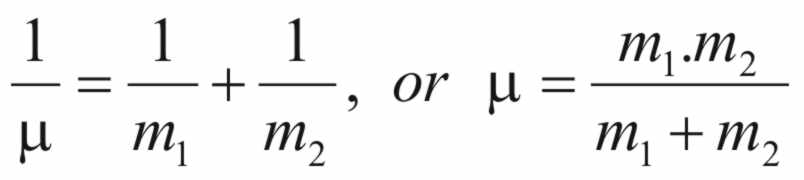 (3)
(3)
Now,
if (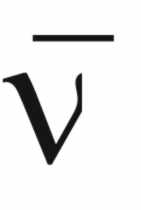 )
is the wave number,
)
is the wave number, 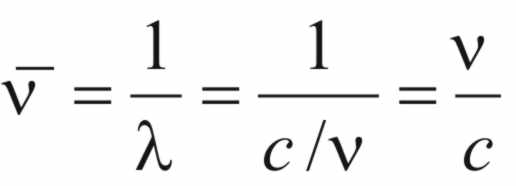 (4)
(4)
Thus,
from (2),
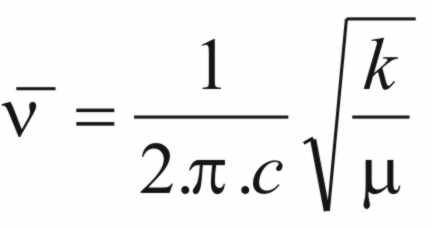 (5)
(5)
Consequently,
a characteristic fundamental frequency and a specific absorption band
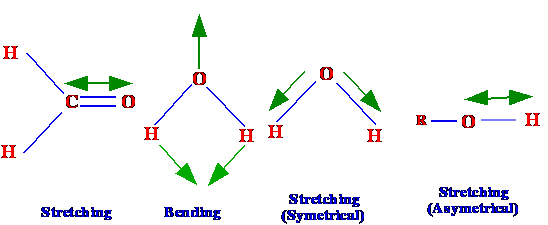
will be associated with each vibration mode. Vibrations can be due to
a change in bond length stretching or alternatively due to a change
in the bond angle bending as shown in figure 4. Depending on the
molecule (cf.
water in figure 3), stretching vibrations can be symmetrical or
asymmetrical.
In
addition, the stretching process can take place in a number of
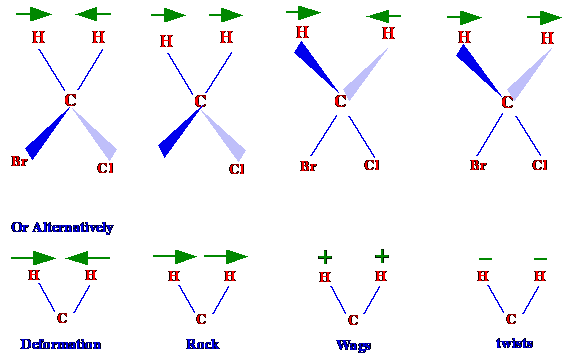
different ways. Consider now the vibrations possible from
bromochloromethane. There are four possible ways the hydrogen -carbon
bond can vibrate. These four possibilities are shown diagrammatically
in figure 5 and are termed deformation, rock, wags and twists. The
two common methods of depicting these processes are included in
figure 5.
Fortunately,
the analysis of complex molecules is simpler than it might appear.
The hydrogen atoms in the molecule can be considered exclusively
because they are often attached to more massive and, thus, the more
rigid parts of the molecule and, as a consequence, will not have a
great effect the hydrogen bond vibrations.
When
m1
>>> m2,
then,
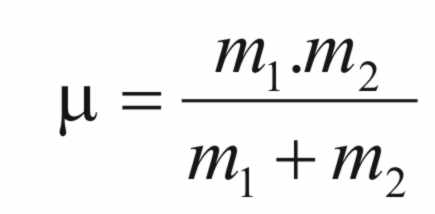 tends to m2,
i.e,
the mass of the hydrogen atom.
tends to m2,
i.e,
the mass of the hydrogen atom.
Both
UV and IR absorption provide spectra that are characteristic of the
molecule and both can be used for compound identification. IR
spectra, however, because of the relatively large number of possible
absorption bands, show considerable differences between diverse
molecules and contain much fine structure. This is in contrast to the
majority of UV spectra that are very similar for many compounds even
though the structure of the molecules may differ considerably. It
follows, that IR spectra can be far more useful for confirming
compound identity than UV spectra. Unfortunately, the measurement of
an IR Spectrum requires considerably more sample than that required
for a UV Spectrum and, thus, although the IR Spectrum is more
informative, the technique is not as sensitive.
IR
spectra can be presented as curves relating Transmission
to, frequency,
wavelength or
wave
numbers;
alternatively the curves can relate Absorbance
to the same variables. Transmission is obviously the complement of
absorption and both methods of presentation are usually available on
IR spectrometers. The Spectrum for polystyrene is shown both as a
transmission curve and an absorption curve in figure 6.
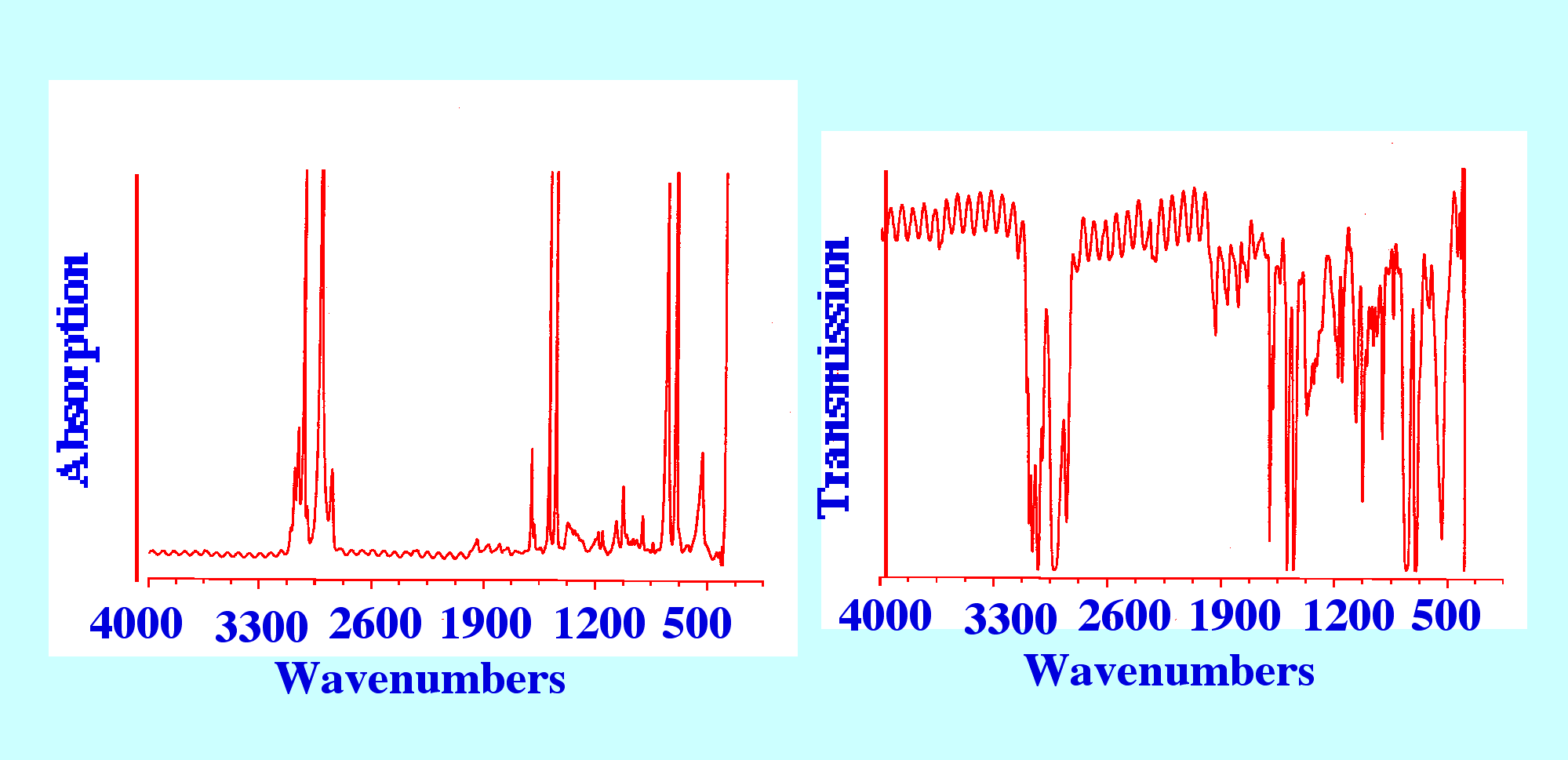
It
is seen that the noise on the transmission Spectrum is far greater
than the noise on the absorption Spectrum and this is generally true
for all spectra. It follows, that it is absorption spectra that are
normally used for analytical purposes.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



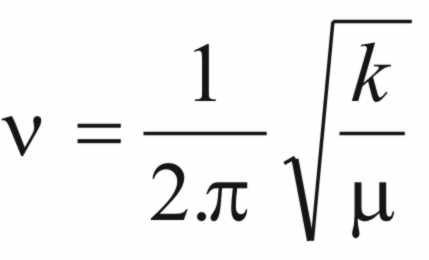 ( 2 )
( 2 )
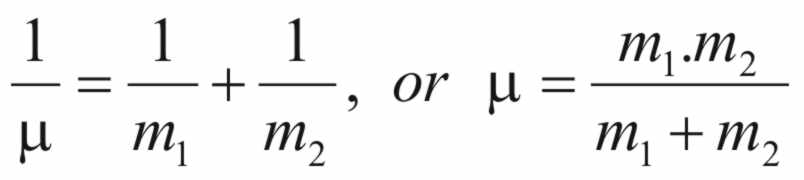 (3)
(3)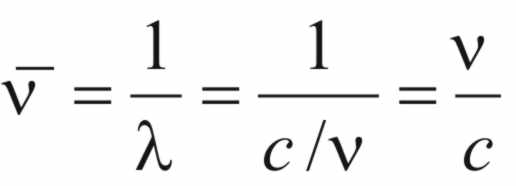 (4)
(4)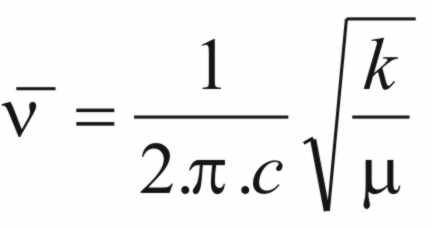 (5)
(5)


