Forms of Light Emission
Light
emitted from an object as a result of its elevated temperature is
called incandescence,
however, light emitted from a body by processes other than high
temperature emission is called Luminescence.
When molecules are excited by electromagnetic radiation to produce
Luminescence, the emitted light is called photoluminescence.
If the release of electromagnetic energy is immediate, or stops on
the removal of the exciting radiation, the substance is said to be fluorescent.If the release of energy is delayed, or persists after the removal of
the exciting radiation, then the substance is said to be phosphorescent.
There
are other forms of Luminescence. Light emitted from a gas discharge
lamp (e.g.
a neon lamp) is called Electroluminescence
and light emitted during radioactive decay is called radio
Luminescence. If
light is emitted by a chemical reaction then this process is called
chemiLuminescence
and if the reaction has a biological origin (e.g.
the firefly) then the light emission is called Bioluminescence.
Thus,
the study of Fluorescence involves photoluminescence where light is
absorbed by a body and re-emitted at a different wavelength, and when
the incident light is arrested the emission of light stops.
Now
the energy
in a quantum of light of frequency ((ν)
is given by,
E = h.ν = hc/λ (1)
Where (c)
is the velocity of light,
(λ)is
the wavelength of the light,
and
(h)
is Planks constant = 6.62 x 10-27
ergs/sec.
The
size of a single quantum of light energy is inconveniently small and
so the energy associated with the transition of (N)
quanta is used where (N)
is Avogadro’s number 6.02 x 1023 (the number of molecules in a gram molecule of the substance). The
energy associated with the
transition of a gram molecule is called an einstein.
Thus, the number of einsteins required to effect a given transition
will vary with the frequency of the radiation. The transition
energies together with the type of transition in addition to other
pertinent data are summarized in table 1.
Table
1 Approximate Values for Quanta Energies to promote certain Reactions
and Transitions.

Analytical
Fluorescence spectroscopy is largely confined to UV and visible
regions of the Spectrum (although Raman emission might also be
considered a form of Fluorescence or vice
versa). The energy
associated with this region is quite high and amounts to about 100
kilogram calories per einstein. Such energies, on absorption, may be
sufficiently high to initiate chemical reaction or cause molecular
break down (e.g.
the effect of sunlight on certain dyestuffs).
When
a molecule adsorbs a quantum of light, an energy transition occurs in
the molecular orientation of the atom or molecule, and the wavelength
at which this happens will be determined by the nature of the
particular transition. The energy is usually dissipated and the
electron eventually returns to its ground state. However, if the
excess energy of the molecule in the higher energy state is not
dissipated rapidly by collisions with other molecules, or by other
means, the electron will return to the ground state with the emission
of electromagnetic radiation in the form of Fluorescence. As some
energy is inevitably lost before the emission occurs, the emitted
fluorescent light is always of a longer wavelength than that absorbed
on excitation (i.e.
the quantum of light has less energy). Excellent discussions on the
theoretical basis of Fluorescence have been given by Guilbault
[3], Udenfriend [4] and Rhys Williams [5].
In the absorption of
light and the emission of Fluorescence light the quantum efficiency
(υE)
is defined as,
υE = (einsteins emitted)/(einsteins absorbed)
= (number of quanta emitted)/(number of quanta absorbed)
Clearly, this ratio can
never exceed unity.
The fluorescent signal
(IF) (that is the fluorescent light emitted) is given by
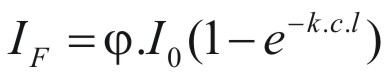 (2)
(2)
|
where (IF)
(φ)
|
is the emitted fluorescent
light
is
quantum yield (the ratio of the number of photons emitted to the
number of photons absorbed),
|
|
(Io)
|
is
the intensity of the incident light,
|
|
(c)
|
is
the concentration of the solute,
|
|
(k)
|
is
the molar absorbance,
|
|
and
(l)
|
is
the path length of the cell.
|
|
|
|

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



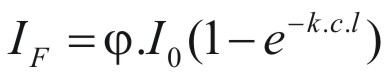 (2)
(2)
