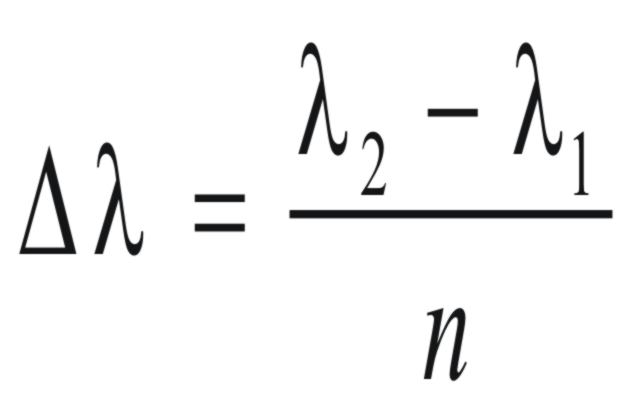ANALYTICAL SPECTROSCOPY
by Raymond P. W. Scott
D.Sc., F.R.S.C., C.Chem., C.Sci. F.A.I.C, F.C.S.
Essential Information for the Analytical Chemist

Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
The Basic UV and Visible Spectrometer
Electromagnetic
radiation can be dispersed into a band of radiation composed of
progressively increasing (or decreasing) wavelength in two ways,
either by means of a suitable prism or by a Diffraction Grating. A
prism consists of a triangular section of material that is
transparent to the light being examined (e.g.
glass for the Visible range of radiation). An example of the
dispersion of white light by a prism is shown in figure 3. A narrow
beam of white light (containing all Visible wavelengths) is made to
strike one surface of the prism at an angle and, on entering the
medium is refracted away from the normal. The refractive index of the
medium differs for light of different wavelength and, thus, the
extent of the light beam bending (the refraction) increases as the
wavelength of the light decreases. Thus, the violet light will be
deflected most and the red light least. As a result the emerging
light will be dispersed into a broad band of colours. Prism have a
larger bandwidth than gratings but gratings are the more popular as
it is easier to obtain high resolution from a grating.
A
diagram of the optical system of a UV/Visible Dispersive Spectrometer
(spectrophotometer). is shown in Figure 8. The components of the
light system through which the light will pass must be made from
quartz or pure silica . In addition, surfaces from which the light is
to be reflected must also have special surface treatment. If very
short wavelengths are to be used then extremely pure silica must be
used for any lens, windows or dispersive units. The deuterium lamp is
used to provide UV Light between 180 and 350 nm and a
tungsten-halogen lamp to provide Visible light between 350 and 800nm.
Light from the source lamp is collimated by two curved mirrors and
focused onto the dispersion unit Diffraction Grating. Originally the
light was dispersed using a quartz prism but all modern instruments
employ a holographic Diffraction Grating. Light of the required
wavelength is selected by adjusting the angle of the grating. The
dispersed light is then focused, by means of a curved mirror, onto a
plane mirror and then focused by means of a lens through the sample
cell. The sample cell must be sufficiently long to provide adequate
light absorption and wide enough to provide enough light to activate
the photocell of the sensor. A micro-cell 1 cm long and 1 mm in
diameter will have a volume of about 8 μl
and a sensitivity of about 1 x 10-8
g/ml (cf
toluene at 254 nm). The exit beam from the sample cell is focused
onto a photo-cell which gives a response that is some function of the
intensity of the light falling on it. The original sensors
that were used took the
form of a photo-cell consisting of a electron emissive surface on
which the light was focused situated in a evacuated bulb fitted with
a second electrode, the anode.

A voltage was
applied cross the electrode system and the electrons emitted by the
light collected and amplified. These devices had limited sensitivity
and were replaced by photo-multipliers, which were similar in form
but contained a staircase of electrodes, the emitted electrons being
amplified at each stage. Modern photocells are usually semiconductor
devices (photodiodes or phototransistors) fitted with pure fused
silica windows. The output from the photocells is not linearly
related to the intensity of the light falling on it and, thus, either
an analogue-modifying amplifier must be used or the response
corrected digitally by a computer.
The wavelength
of the light passing through the sample is selected or scanned by
rotating the grating. The output from the sensor amplifier is
presented on a chart recorder or computer printer as an adsorption
curve relating adsorption to wavelength (or frequency or wave
numbers). The adsorption curve is known as the adsorption Spectrum
and its shape will be characteristic for the substance being
examined.
The Diode Array
UV/Visible Spectrometer
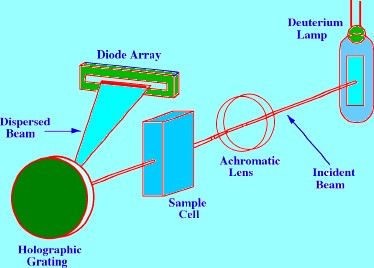
The
Diode Array spectrometer functions in an entirely different way to
that of the dispersive instrument. A diagram of a Diode Array
detector is shown in Figure 7. In the diode
array spectrometer the sample is subjected to light of all
wavelengths generated by the lamp. Light from a deuterium lamp is
collimated by an achromatic lens system so that the total light of
all wavelengths passes through the sample cell onto a holographic
grating. The dispersed light from the grating is then focused onto a
Diode Array. The array may contain many hundreds of diodes and the
output from each diode is regularly sampled by a computer and stored
on disk. The Spectrum of the sample can be obtained by recalling from
memory the output of each of the diodes, i.e.
a curve relating adsorption to wavelength. The only disadvantage of
this type of spectrometer is that the number of diodes in the array
limits its resolution.
If
the resolution of the detector is (Δλ)
will the number of diodes in the array (n), and the range of
wavelengths covered by the array is (λ2 - λ1),
Thus,
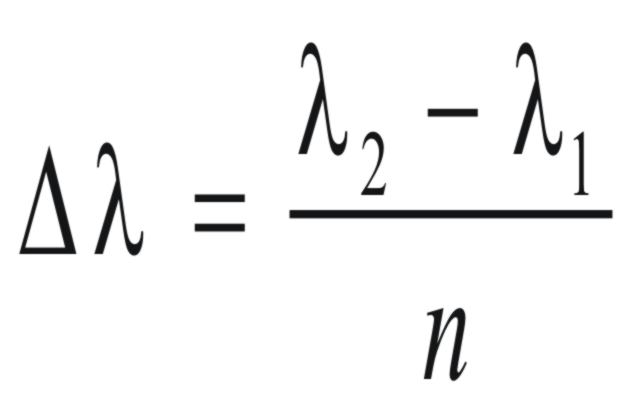
It
is seen that the ultimate resolving power of the Diode Array
detector will depend on the
semi-conductor manufacturer and on how narrow the individual photo
cells can be commercially fabricated. As a result of considerable
research in diode sensors, the size of the diodes are continually
being reduced and consequently the resolution is now very high and
still improving.
Although, the
two spectroscopic systems described appear to be satisfactory, both
suffer from certain disadvantages due to second-order effects. When
light having a narrow band wavelengths is passed through the cell (as
in the dispersive spectrometer) and the whole of the transmitted
light allowed to fall on the sensor, then the light received will not
only be that light of the same wavelength that was transmitted
through the cell, but also any fluorescent light that the incident
light may have excited. Thus, the light measured may not be solely
transmitted light of the selected wavelength but will also contain
any fluorescent light that was generated by the incident light
passing through the sample.
In a similar
way, when light containing the whole range of wavelengths is passed
through the cell (as in the Diode Array spectrometer), then the light
sensed at a selected wavelength will not only contain the transmitted
light of that wavelength but also any fluorescent light of the same
wavelength that may have been excited by incident light of other
wavelengths.
It is seen that
neither spectrometer system can be certain of measuring true light
absorption at a specific wavelength. In fact, true light absorption
can only be measured by first selecting the incident wavelength and
then allowing the transmitted light to be resolved on another
monochromator. The second monochromator selects the same wavelength
as the incident light and in this way any fluorescent light is
eliminated and only the transmitted light measured.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.