The Particle Beam Interface
The particle beam interface
involves
nebulizing the sample solution, and the solvent free particles of
solute are then passed into the ionization chamber of the mass
spectrometer. Electron impact or Chemical Ionization spectra can be
produced, and the system has been given name the of the somewhat
vainglorious title of the monodisperse
aerosol generation sampling interface. In
addition, it has
been endowed with the very pretentious acronym (MAGIC). Willoughby
and Browner [37] and Winkler et al. [38] were two
of the early
groups working on this interface, which consists of two parts, the
aerosol generator and the momentum separator. A diagram of the
aerosol generator developed by Winkler et al. is
shown in
figure 59.
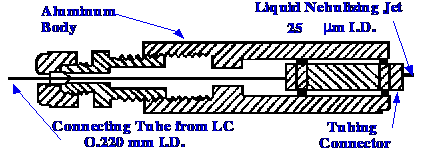
Nebulization takes place at the
end of a
fused silica tube, 25 μm
I.D., similar tubing employed in capillary gas chromatography. The
short length of fused silica tubing is connected to the conduit tube
from the LC column (220 μm
I.D.) by a low dead volume fused silica tubing connector. The liquid
jet forms at the end of the small-diameter tubing, and was found to
be very efficient and seldom clogged. Nevertheless, precautions must
be taken to ensure that no solid particles are carried into the
conduit from the column. A diagram of the second part of the
interface, the momentum separator, is shown in figure 60.
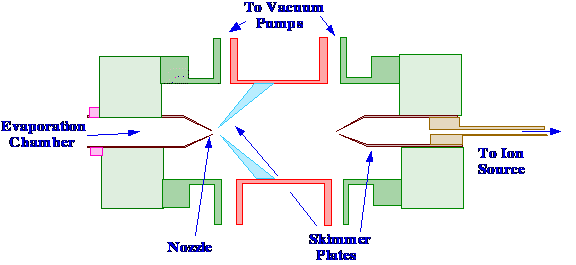
In previous work [39[ [40] the
most
important sources of sample loss from this type of interface were
found to be particle sedimentation, poor nozzle/skimmer alignment and
loss due to turbulence. Consequently, the skimmers were designed to
provide an undisturbed path from their point of generation, until
they were in the ion source of the mass spectrometer. The body of the
momentum separator was made of stainless steel and the nozzles and
skimmers were machined from 6010 grade aluminum. The separation
between the nozzle and the first skimmer was about 10 mm and was
adjustable by the use of shims. The first skimmer had a 100˚
exterior angle and a 95˚ interior angle with a 0.5 mm orifice.
The second skimmer had a 45˚ exterior angle and a 30˚
interior angle and a 1 mm orifice. The distance between the two
skimmers was also about 10 mm and was also adjustable. Both interlock
chambers were pumped with mechanical vane (hot oil) pumps, having a
capacity of 21.6 m3/h.
The interface was found to be
easy to
operate, the skimmer provided little turbulence and it had high
transport efficiency. The device was tested by using the normal phase
separation of the cis and trans
isomers of retinol
acetate. The separation obtained is shown in figure 61.
The total mass of sample
injected was 50 ng
and the chromatogram has not been smoothed in any way. It is seen
that the separation does not appear to be impaired by the interface
and the sensitivity that is achieved, compares well with other types
of interface. Another example of the use of the interface in the
separation of some aliphatic fatty acids shown in figure 61.
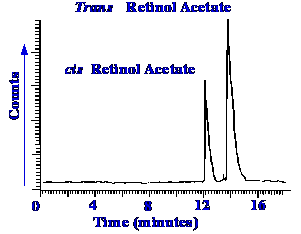
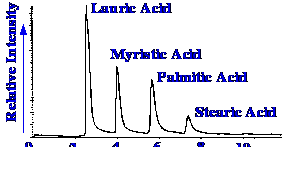
Figure 62. The Total
Ion Current
Chromatogram of Some Mixed Fatty Acids
In
this example a reversed phase column was employed with gradient
development from 80% methanol and 20% water (containing 3% acetic
acid) and 100% methanol . It is seen that there is some indication of
tailing, although whether this arises from the column or the
interface is not clear. It is also seen that the nebulizer functions
well with aqueous solution containing 20% of water. It is not
reported whether or not higher water contents work equally as well.
The electron impact Spectrum obtained for lauric acid is shown in
figure 63.
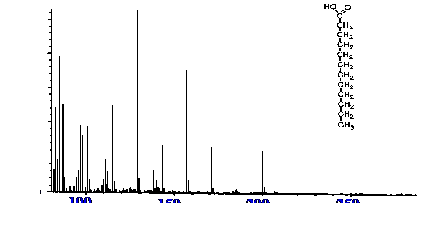
The
EI Spectrum exhibits a significant molecular ion and agrees
completely with the reference spectra of that compound. It should be
noted that the Spectrum is presented without background subtraction.
There is no evidence of thermal degradation in the interface, despite
the relatively high temperature of the ion source (240˚C).
Cappiello and Famiglini [41]
extended the
work and designed a micro-flow particle beam interface. They started
with the Particle Beam Interface manufactured by Hewlett-Packard,
(similar in form to the device described previously) and modified the
nebulizer to operate at lower flow rates. The basic design is shown
in figure 64. The end portion of the coaxial tubing, carrying the
helium, was widened to keep any slow-growing liquid droplets away
from the internal gas conduit wall. This enlargement is sharply
reduced at the capillary tip, thus keeping the contact surface
between the capillary and the wall of the gas conduit to a minimum.
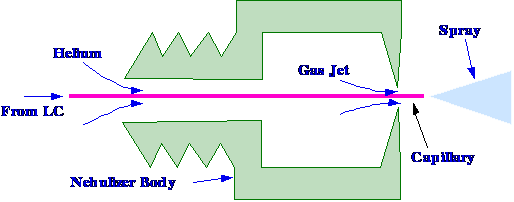
The unique character of this
device is its
capacity to nebulize very small liquid flows. In the absence of a
helium flow, the liquid is not forced out by the following liquid but
remains as an expanding droplet. When the helium flow is commenced,
as soon as the droplet size exceeds the diameter of the tip, the
energy of the gas breaks the surface tension forces and droplets are
formed. An example of the performance of the interface is shown in
figure 65.
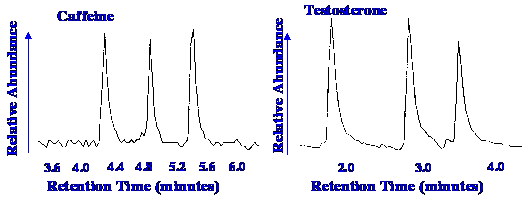
The peaks in Figure 65 represent
the
injection of three samples of 40 pg of caffeine (m/z of monitoring
ion of 194) and three samples of 600 pg of Testosterone (m/z of
monitoring ion 124). The flow rate was 2 μl/min.
In general, the authors claimed very little nebulizer contamination
over long periods of time, better overall performance over a wide
range of mobile phase composition, efficient nebulization over a wide
range of flow rates and more simple operational procedures.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.









