
Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Introduction to Infrared Spectroscopy
Infrared
spectroscopy is probably the most common spectroscopic technique
employed by chemists today and in particular analytical chemists.
There are a number of reasons for its popularity. It can be used in
qualitative analysis to identify specific substances for which there
are reference spectra, it can be used to aid in structure elucidation
and, in particular identify specific chemical groups present in a
compound. In certain instances it can also be employed for
quantitative analysis.


In
addition, the infrared spectrometer is relatively inexpensive,
compact in size, so it can stand on a laboratory bench without taking
up too much room, is easy to operate and produces spectra in a few
minutes or less. The technique is not as sensitive as UV/vis or mass
spectrometry but its sensitivity is more than adequate for most
analytical purposes.
IR
spectroscopy involves the absorption of light having a wavelength
longer than the Visible Spectrum, i.e.
between 2 and 15 micron (1.5x1016
Herz to 1.8x1015
Herz).
In
practice, whereas UV/Visible spectra are usually represented as
curves relating transmission or absorbance against wavelength, in
infrared spectroscopy the independent variable is usually measured as
wave numbers, as opposed to wavelength.
The
wave number for a particular wave having frequency (ν)
is taken as (1/λ)
which is the number of waves per centimeter.
Bearing
in mind that E =h.ν and c = ν.λ,
where
(E)
is the energy of the photon, (ν)
is the frequency of the radiation, (λ)
is the wavelength of the radiation, and
(h)
is Planks Constant.
If
(Δ)
is the wave number of a photon of wavelength (λ)
Then
Δ = (1/λ) = (ν/c) = E/hc
and is expressed in reciprocal centimetres (cm -1).
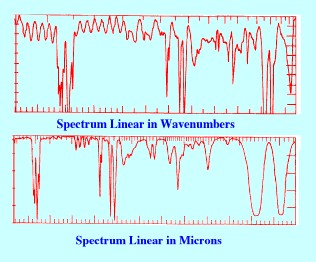
Both
forms of Spectrum presentation (i.e.
curves relating absorption against wavelength
and wave number) are shown in Figure 2 and it is seen that the
appearance of each Spectrum by the two methods is very different.
The
infrared band of frequencies can be divided roughly into three groups
of interest to the analyst or practicing chemist. The three groups
are given in the following table. Of the three groups the group
dealing with fundamental rotational-vibration energies have, so far,
be found the most useful to the practical chemist.
| Region |
Wavenumber Range |
wavelength Range |
| |
cm-1 |
microns |
| Near Infra Red (overtones |
13,300 - 4000 |
0.75 - 2.5 |
| Fundamental Rotation Vibration |
4000 - 400 |
2.5 - 25 |
| Far Infrared (skeletal vibrations) |
400 - 20 |
26-500 |
Infrared
spectroscopy has been shown to be a particularly valuable aid to the
organic chemists. Organic substances exhibit explicit absorption
characteristics to radiation of specific frequencies that can be
exclusive for a particular substance. In addition, the Spectrum of a
mixture usually constitutes a Spectrum addition in that it contains
the spectra of both substances superimposed. Finally the intensity of
the absorption at any particular wavelength is related to the
quantity of the substance present in the sample.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.






