Diffuse Reflectance IR Fourier Transform Spectrometry (DRIFTS)
The
principle of Diffuse Reflectance IR Fourier transform spectrometry is
depicted in figure 20.
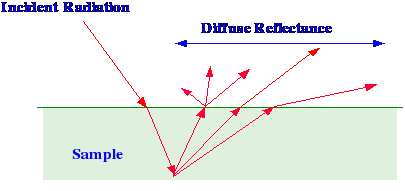
When
incident light strikes a surface, the light that penetrates is
reflected in all directions and this is called Diffuse Reflectance.
As the light that leaves the surface has passed through a thin layer
of the reflecting material, its wavelength content will have been
modified by the optical properties of the matrix. Consequently, the
wavelength and intensity distribution of the reflected light will
contain structural information on the substrate. It is clear that
this process would be applicable to the study of surfaces and
coatings and could obviously be employed to scan TLC plates. The use
of the technique for scanning TLC plates was investigated by Zuber et
al. [2].
The
TLC plates were inserted directly into the spectrometer sample
chamber and the Spectrum obtained by reflectance directly from the
plate surface. A reference laser was used to aid in spot alignment,
and the plate contributions to the background were subtracted from
the Spectrum, which was obtained in the usual manner. It was found
that spot identification was possible, providing
reference spectra were available that had been obtained under the
same operating conditions.
The quality of the spectra and the useful size of the IR window
available varied between different types of plate.
The
preparation and care in handling both the sample and the background
plates were extremely important to the success of the method.
Although solvent selection was critical, providing the plate was
completely dry, the solvent had no effect on the quality of the
spectra produced. The Nicolet (Madison, WI) 6000 FTIR spectrometer
equipped with a nitrogen cooled cadmium telluride detector was
employed, and a Diffuse Reflectance attachment was used to run the
spectra.
The
interferometer was run at a mirror velocity of 0.586 cm/s and 2000
scans or less was found necessary to produce a good quality TLC/FTIR
Spectrum. The TLC spot diameter varied between 2 and 8 mm, and the
diameter of the infrared beam, focused on each plate, was 1 mm. The
sample and background spectra were run, and the Spectrum of the
sample obtained by difference. The analysis time was typically 15
minutes for the TLC separation, and about 30 minutes were needed to
obtain the IR spectra. Examples of the results obtained from the
tandem system are shown in figure 21.
It
is seen that there is a distinct difference in the form of the
spectra taken from the TLC plate compared with that from the KBr
pellet. It follows that reference spectra that are to be used for
solute identification should also be obtained from the TLC plate in
the same manner. The mass of solute in each spot examined was about
10 μg but it was
estimated that about 1 μg
would be sufficient for a recognizable Spectrum to be obtained.
Direct
measurements taken on the plate restricts the range of wavelengths
that can be employed in the spectroscopic examination, whereas the
removal of the solute from
the plate allows the material to be examined over the normal range of
wavelengths. Unfortunately, solute removal and recovery almost always
involves losses, and sometimes the losses are accompanied by the
decomposition or molecular rearrangement of labile materials.
Chalmers
et al. [4] chose to use FTIR Diffuse Reflectance spectrometry in an
off-line manner, by extracting the material from the spot before
measurement.
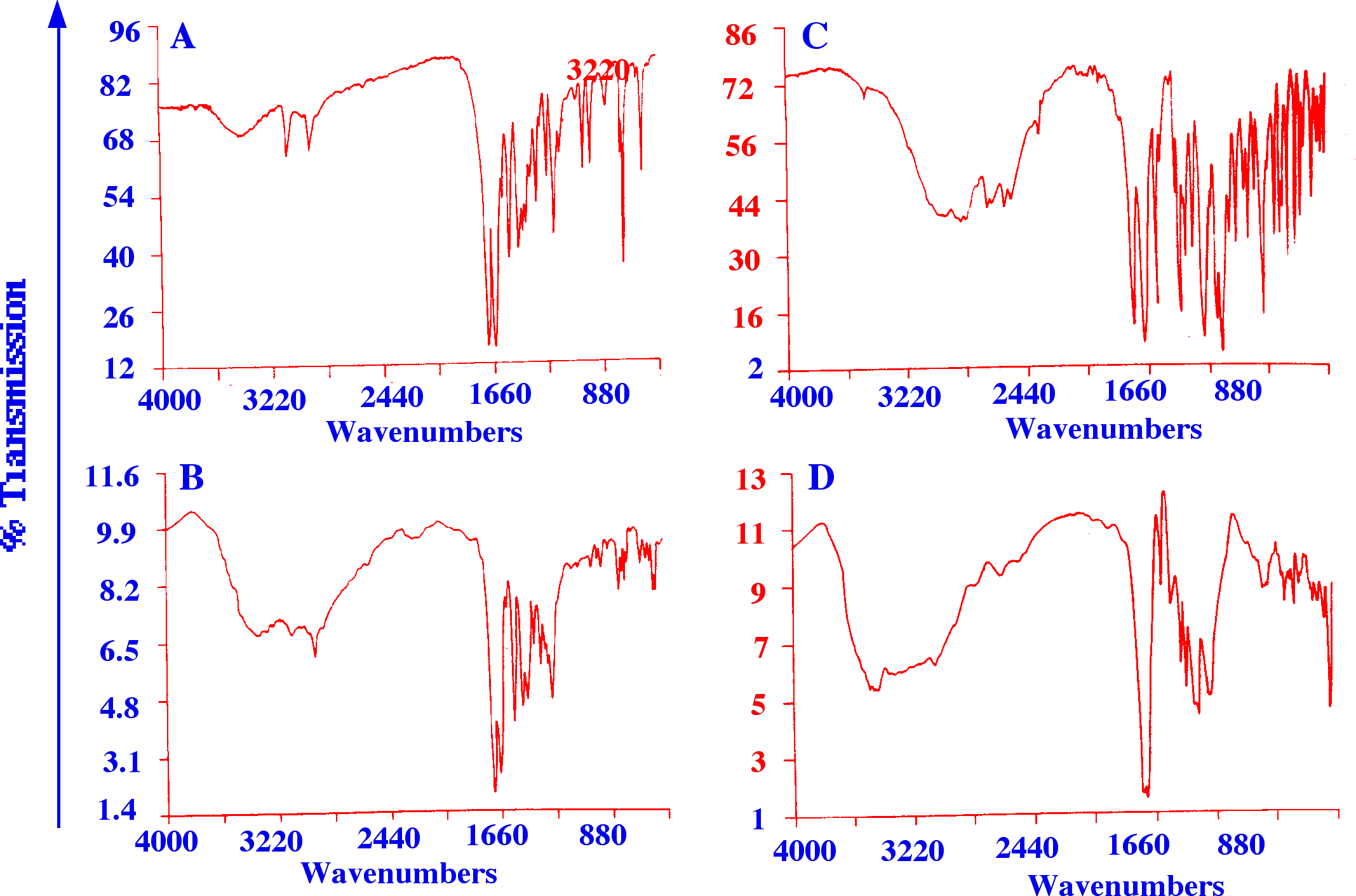
A
and C, Spectra from KBr pellets of caffeine and aspirin respectively.
B and D, Reflectance spectra from TLC plates of caffeine and aspirin
respectively.
The
solute on the TLC plate was transferred to a KCl pellet, made
directly from ball-milled potassium chloride. 0.7 g of the dried
powder was pressed into a 13 mm disk die, at a pressure of 500 psi.
The resulting pellet was about 4 mm high (± 0.5 mm). A metal
backed TLC plate was employed for the separation, and the spot cut
out and placed, metal backing downwards, in a tube 5 cm long and 17
mm I.D..
The
KCl pellet was placed on the top of the disc, and about 2 ml of
chloroform carefully pipetted down the inside of the tube.
The
chloroform was allowed to evaporate at room temperature, and after
about an hour, the pellets were removed for IR examination. An
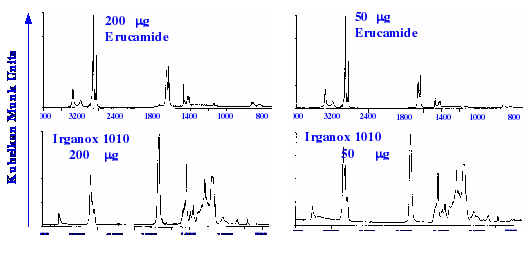
example of the spectra taken of some samples provided by the plastics
industry is shown in figure 22. It is seen that good spectra were
obtained, but the process was tedious, and considering the extraction
and concentration processes that were involved, the overall
methodology does not appear to have provided a very good sensitivity.
.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.





