
Specialising in custom-designed, precision scientific instruments, built, programmed and calibrated
to the most exacting standards. The range includes precision dataloging barographs,
with built-in statistical analysis, Barographic Transient Event Recorders
and computer-interfaced detectors and sensors
for environmental monitoring & process control.

A site dedicated to scientific techniques, experimental methods, &
investigative tools for the inventor, researcher
and laboratory pioneer. Articles on glassblowing, electronics, metalcasting, magnetic
measurements with new material added continually. Check it out!
www.drkfs.net
Basically,
the components of a Raman spectrometer comprise a light source, some
collimating optics, a dispersing element such as a diffraction
grating and a light sensor. The major problem associated with Raman
spectroscopy is the very weak intensity of the scattered light, which
virtually demands an extremely high-energy light source such as a
laser. It follows, that the spectrometer must also have very high
discrimination against internal scattered or stray light and the
detecting device must have a very high sensitivity.
Originally
there were basically two types of laser source for Raman Spectroscopy
the Continuous Wave source and
the pulsed gas ion laser source. Raman
spectrometers originally utilized continuous
wave argon or krypton gas
lasers. The gas used was important as the Raman Scattering cross
section varies as the fourth power of the light frequency. Thus,
spectra obtained at 514.5 nm from an argon laser was from 2.3 to 4.5
times more intense than spectra obtained at 632.6 nm from a He-Ne
laser operating at the same power. For additional power in the red
region of the Visible Spectrum, dye lasers (pumped with a high power
argon laser) were also employed to complement the basic gas lasers.
Pulsed lasers in Raman spectrometers are not so common. The frequency
doubled Nd/YAG laser with a fundamental at 1.06mm was used because it
was powerful, stable and reliable. Although it can be used in the
continuous mode it was more often used in the pulsed mode with pulse
widths of 10ns and a peak power output of 500kW. Employing potassium
tri-hydrogen phosphate and potassium di-hydrogen phosphate outputs of
532.0, 354.6 and 266,0 nm could be obtained. A synchronously pumped
dye laser can also be used as a pulsed output system. Pulsed
lasers were mostly employed for UV Raman measurements.
Today
diode
lasers are
commonly employed to produce excitation units in
Raman Spectroscopy
and diode lasers having powers of 30 to 300 mW are readily available
providing light at 785 and 830 nm. Tuneable lasers providing
radiation between 670nm to 1100 nm using Ti sapphire lasers are also
readily available. The great advantage of the diode laser is that it
is monochromatic. The power used for the lasers, however, must be
carefully controlled so as not to degrade the sample.
If
the laser is not a solid state device it is usually focused on the
sample using a long focal length lens that is anti-reflection coated
for the laser line of interest. The spot size at the sample can be
adjusted by changing the focal length of the lens. To simplify the
collection process, the spot size is made as small as possible (if a
solid state laser is used this, of course is unnecessary). In
addition to the focusing lens, a polarization rotator and a line
filter are usually incorporated. When used, the laser line filter is
either a narrow band pass interference filter or a prism
monochromator. The filter must have a high transmission at the
selected laser line and high rejection at other wavelengths (more
than 99.9% rejection).
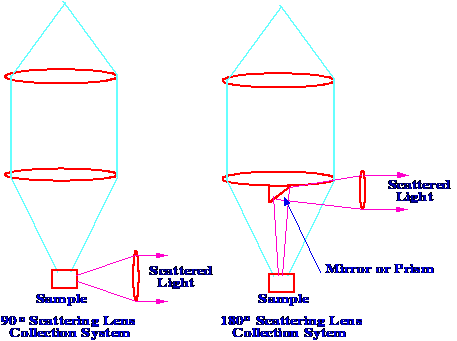
The
90o
and 180o
Lens Collection Systems
The
collection system for the Raman scattered light comprises of two
lenses the first a very short focal length with a low f
number to collect the largest possible solid angle and the second
focuses the collected light onto the entrance slit of a monchromator.
Most instruments operate with a 90o
or 180o
scattering geometry. The 90o
system is the easiest to operate as the since the illumination axis
and the collection axis are apart in space. The 90o
system can be modified to the 180o
system by placing a small mirror prism on the face of the first lens.
The two systems are shown diagrammatically in figure 5.
The
monochromator must disperse the scattered light across a slit for
sequential presentation to the detector or across a solid-state diode
array. The standard dispersing device in modern Raman spectrometers
is the double monochromator or the dual grating system. The
disadvantage of this system is the transmission loss and, as a
consequence, the Diode Array system is now the more popular.
The
single channel sensor system was originally the photomultiplier tube
that must be designed to have an extended red response and a very low
dark count. Direct current amplitude detection is now hardly ever
used and photon counting systems now have a large dynamic range and
can, thus, deal with a wide range of scattered light intensities.
The
multi-channel sensor system utilizes a Diode Array with a signal
intensifier coupled to it. The only disadvantage of this system is
that the resolution depends on the number of diodes and thus, the
size of the diodes. However, with modern solid-state technology, the
diodes can be made very narrow and, thus, the resolution fairly high.

About the Author
RAYMOND PETER WILLIAM SCOTT was born on June 20 1924 in Erith, Kent, UK. He studied at the
University of London, obtaining his B.Sc. degree in 1946 and his D.Sc. degree in 1960.
After spending more than a decade at Benzole Producers, Ltd. Where he became head of
the Physical Chemistry Laboratory, he moved to Unilever Research Laboratories as
Manager of their Physical Chemistry department. In 1969 he became Director of Physical
Chemistry at Hoffmann-La Roche, Nutley, NJ, U.S.A. and subsequently accepted the position
of Director of the Applied Research Department at the Perkin-Elmer Corporation, Norwalk, CT, U.S.A.
In 1986 he became an independent consultant and was appointed Visiting Professor at Georgetown
University, Washington, DC, U.S.A. and at Berkbeck College of the University of London; in 1986
he retired but continues to write technical books dealing with various aspects of physical chemistry
and physical chemical techniques. Dr. Scott has authored or co-authored over 200 peer reviewed
scientific papers and authored, co-authored or edited over thirty books on various aspects of
physical and analytical chemistry. Dr. Scott was a founding member of the British chromatography
Society and received the American Chemical society Award in chromatography (1977), the
M. S. Tswett chromatography Medal (1978), the Tswett chromatography Medal U.S.S.R., (1979),
the A. J. P. Martin chromatography Award (1982) and the Royal Society of Chemistry Award in
Analysis and Instrumentation (1988).
Dr. Scott’s activities in gas chromatography started at the inception of the technique,
inventing the Heat of Combustion Detector (the precursor of the Flame Ionization Detector),
pioneered work on high sensitivity detectors, high efficiency columns and presented fundamental
treatments of the relationship between the theory and practice of the technique.
He established the viability of the moving bed continuous preparative gas chromatography,
examined both theoretically and experimentally those factors that controlled dispersion
in packed beds and helped establish the gas chromatograph as a process monitoring instrument.
Dr. Scott took and active part in the renaissance of liquid chromatography,
was involved in the development of high performance liquid chromatography and invented
the wire transport detector. He invented the liquid chromatography mass spectrometry
transport interface, introduced micro-bore liquid chromatography columns and used them
to provide columns of 750,000 theoretical plates and liquid chromatography separations
in less than a second.
Dr. Scott has always been a “hands-on” scientist with a remarkable record of accomplishments in chromatography ranging from hardware design to the development of fundamental theory. He has never shied away from questioning “conventional wisdom” and his original approach to problems has often produced significant breakthroughs.



